Shilpa Medicare Walk-In QC, IPM, RA (RoW/US/EU/CA)

- Join Shilpa Medicare: Hiring for Quality Control, Regulatory Affairs, and IPM Roles in Hyderabad
- Departments & Job Openings
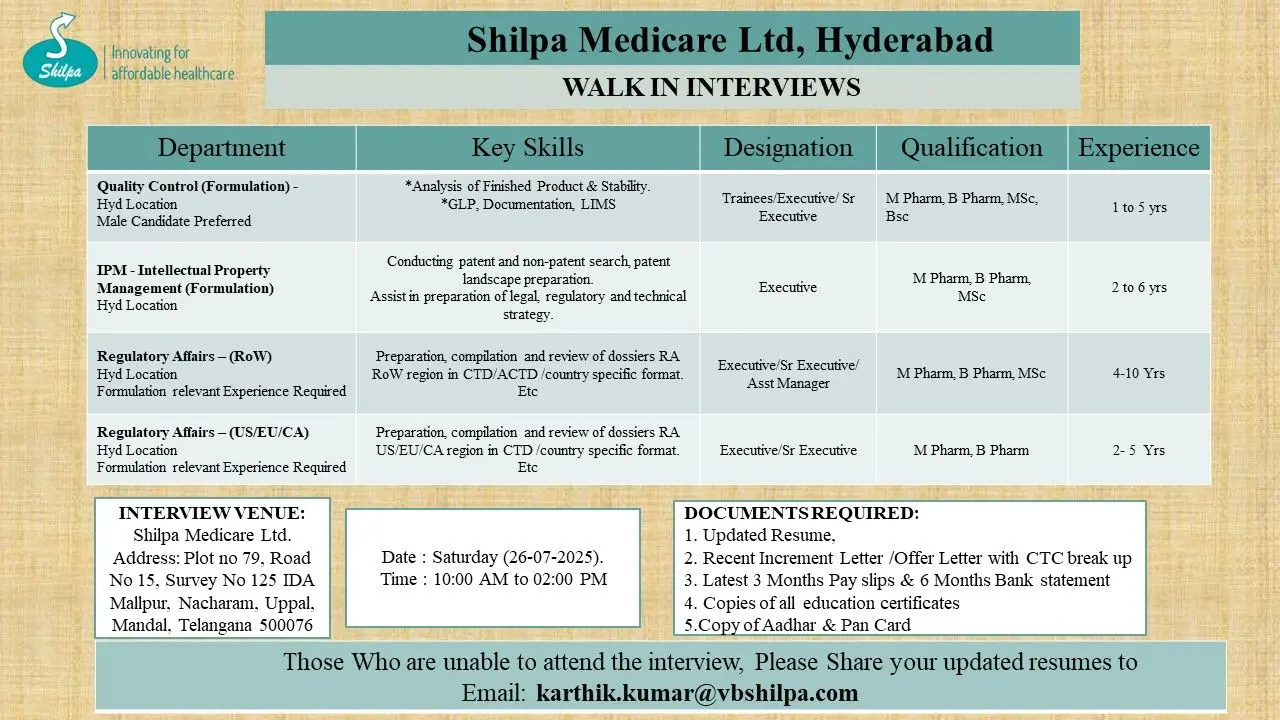
- Quality Control (Formulation) – Hyderabad
- Intellectual Property Management (IPM) – Hyderabad
- Regulatory Affairs (RoW) – Hyderabad
- Regulatory Affairs (US/EU/CA) – Hyderabad
- Documents to Carry
- Can’t Attend the Walk-In?
- Why Join Shilpa Medicare?
- Job Summary Table
Shilpa Medicare Hiring B Pharm/M.Pharm/MSc Graduates for QC, Regulatory Affairs & IPM Roles in Hyderabad
Explore current pharma job openings at Shilpa Medicare Hyderabad for B Pharm, M.Pharm, and MSc graduates in QC, Regulatory Affairs, and IPM with 1–10 years of experience.
Join Shilpa Medicare: Hiring for Quality Control, Regulatory Affairs, and IPM Roles in Hyderabad
Shilpa Medicare Ltd, a leading name in innovative and affordable healthcare, is conducting walk-in interviews in Hyderabad for multiple pharma roles. If you’re a B Pharm, M.Pharm, MSc, or BSc graduate with 1–10 years of relevant experience, this is your opportunity to grow in a reputed and regulatory-compliant pharmaceutical environment.
Walk-In Date: Saturday, 26 July 2025
Time: 10:00 AM to 2:00 PM
Location: Plot No. 79, Road No. 15, Survey No. 125, IDA Mallapur, Nacharam, Uppal Mandal, Telangana – 500076
Departments & Job Openings
Quality Control (Formulation) – Hyderabad
Designation: Trainee / Executive / Sr. Executive
Eligibility: B Pharm / M.Pharm / MSc / BSc
Experience: 1–5 years
Key Skills:
- Finished Product & Stability Analysis
- Good Laboratory Practices (GLP)
- Documentation and LIMS systems
Note: Male candidates are preferred for this role.
Intellectual Property Management (IPM) – Hyderabad
Designation: Executive
Eligibility: M.Pharm / B Pharm / MSc
Experience: 2–6 years
Key Responsibilities:
- Patent and non-patent search
- Preparing patent landscapes
- Support in legal and regulatory strategy formulation
Regulatory Affairs (RoW) – Hyderabad
Designation: Executive / Sr. Executive / Assistant Manager
Eligibility: M.Pharm / B Pharm / MSc
Experience: 4–10 years
Job Profile:
- Compilation of dossiers for RoW markets in CTD/ACTD formats
- Experience in formulation-related submissions
Regulatory Affairs (US/EU/CA) – Hyderabad
Designation: Executive / Sr. Executive
Eligibility: M.Pharm / B Pharm
Experience: 2–5 years
Job Role:
- Preparation and review of regulatory submissions for US, EU, and Canada
- Familiarity with CTD and country-specific formats
Documents to Carry
Candidates attending the walk-in must bring:
- Updated Resume
- Recent Increment/Offer Letter with CTC breakup
- Last 3 months’ payslips and 6 months’ bank statement
- Copies of all academic certificates
- PAN and Aadhar card copies
Can’t Attend the Walk-In?
No worries. You can email your updated resume to: karthik.kumar@vbshilpa.com
Why Join Shilpa Medicare?
- Exposure to regulated market standards (US/EU/CA)
- Opportunities in both formulation development and regulatory management
- Career growth with a leading pharma company committed to affordable healthcare
Job Summary Table
| Company Name | Shilpa Medicare Ltd |
|---|---|
| Vacancies | QC, IPM, RA (RoW/US/EU/CA) |
| Required Education | B Pharm, M.Pharm, MSc, BSc |
| Experience Required | 1 to 10 Years |
| Job Location | Hyderabad, Telangana |