Lactose India Hiring Officer, Sr. Officer & Executive

- Company Overview
- Job Role & Responsibilities
- Eligibility / Qualifications
- Location & Salary
- Application Process
- Why Join Lactose India Limited?
- FAQs
- Summary Table
B.Pharm, M.Pharm, BSc, MSc – Multiple Openings | Lactose India Ltd Vadodara
Apply now for Officer, Sr. Officer & Executive roles at Lactose India Ltd, Vadodara. B.Pharm, M.Pharm, BSc, MSc candidates eligible. Freshers welcome!
Lactose India Limited, a reputed and globally recognized pharmaceutical manufacturing company, is inviting skilled and enthusiastic professionals to join its dynamic team across multiple departments. Located in Poicha (Rania), Taluka Savli, District Vadodara, this WHO–GMP certified facility offers excellent career growth opportunities for both freshers and experienced candidates.
Company Overview
Lactose India Limited is a trusted name in the pharmaceutical industry, known for its commitment to innovation, quality, and sustainable healthcare manufacturing. With a strong presence in the domestic and international markets, the company continues to expand its product portfolio, focusing on Active Pharmaceutical Ingredients (API), Oral Solid Dosage (OSD) formulations, and industrial chemical solutions.
The organization’s success stems from its skilled workforce, advanced manufacturing infrastructure, and adherence to global quality and environmental standards. Working at Lactose India Limited means being part of a culture that values learning, innovation, and continuous improvement.
Job Role & Responsibilities
Lactose India Limited is currently hiring for multiple departments, offering exciting opportunities for candidates with backgrounds in Pharmacy, Science, and Engineering. Below are the available positions and key responsibilities:
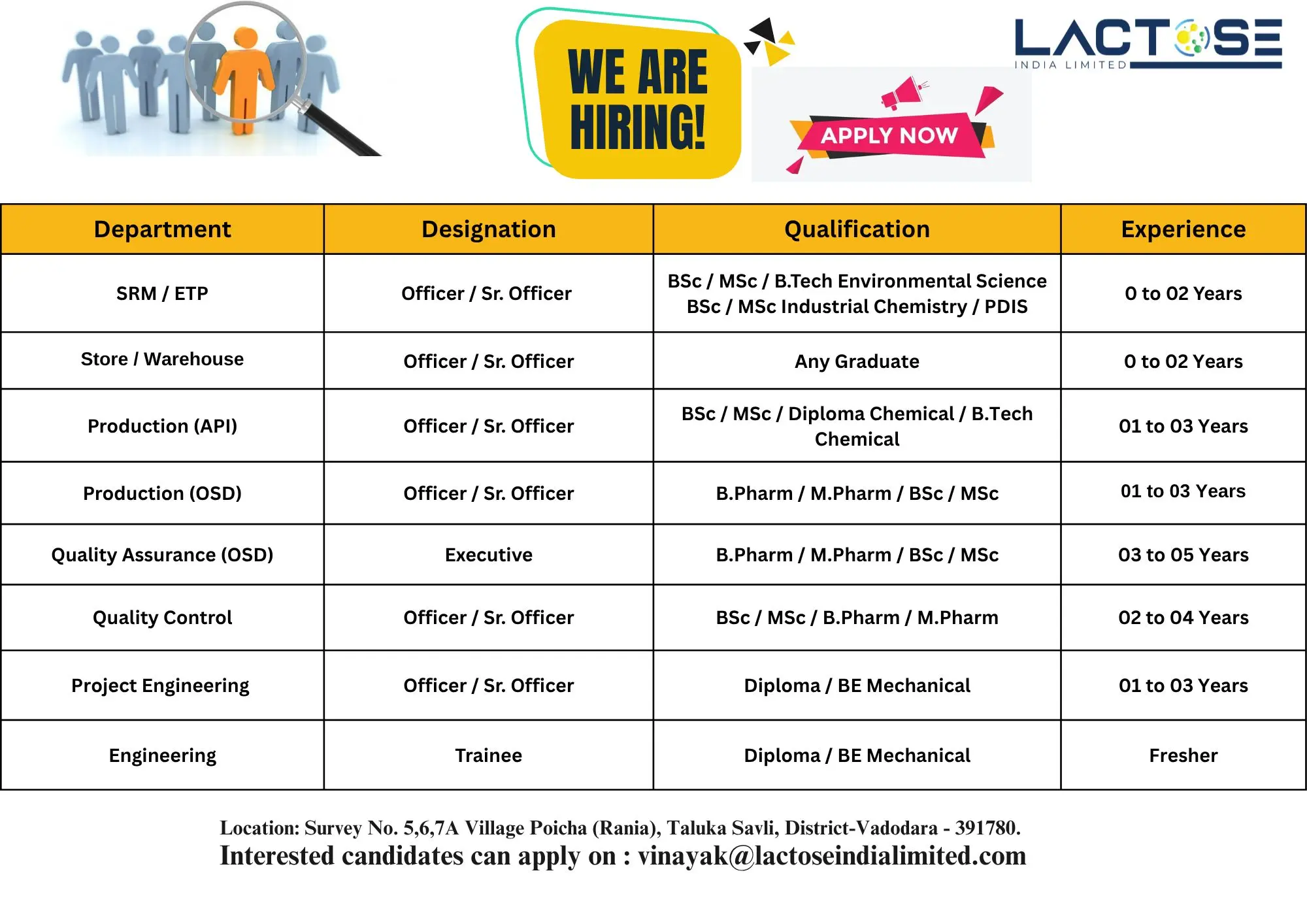
1. SRM / ETP – Officer / Sr. Officer
- Perform operations and monitoring of the Effluent Treatment Plant (ETP).
- Ensure compliance with environmental standards and safety protocols.
- Prepare daily reports and documentation as per company SOPs.
2. Store / Warehouse – Officer / Sr. Officer
- Manage inventory, material handling, and stock records.
- Maintain GRN, issue slips, and ensure FIFO/LIFO compliance.
- Coordinate with purchase and production teams for timely dispatch and receipt.
3. Production (API) – Officer / Sr. Officer
- Operate and monitor production equipment in the API manufacturing unit.
- Ensure batch completion as per BMR/BPR guidelines.
- Maintain records and support GMP documentation.
4. Production (OSD) – Officer / Sr. Officer
- Supervise tablet and capsule manufacturing processes.
- Maintain compliance with cGMP and regulatory guidelines.
- Support process validation and documentation review.
5. Quality Assurance (OSD) – Executive
- Conduct line clearance, in-process checks, and deviation management.
- Review BMRs/BPRs and ensure documentation accuracy.
- Ensure adherence to SOPs, cGMP, and audit readiness.
6. Quality Control – Officer / Sr. Officer
- Perform testing of raw materials, in-process samples, and finished products.
- Handle HPLC, GC, and wet analysis.
- Maintain laboratory records in compliance with GLP.
7. Project Engineering – Officer / Sr. Officer
- Handle preventive maintenance and calibration of equipment.
- Support new project installations and mechanical engineering tasks.
- Coordinate with vendors and ensure adherence to engineering standards.
8. Engineering – Trainee
- Assist in daily maintenance and utility operations.
- Learn and support the technical team in routine maintenance.
- Excellent opportunity for Diploma / BE Mechanical freshers to gain hands-on experience.
Eligibility / Qualifications
- Education: B.Pharm, M.Pharm, BSc, MSc, Diploma Chemical, B.Tech Chemical, Diploma / BE Mechanical, BSc / MSc Industrial Chemistry, BSc / MSc Environmental Science, PDIS.
- Experience: 0–5 years (Freshers can apply for trainee roles).
- Skills Required: Good knowledge of GMP, documentation, and safety compliance. Strong communication and teamwork abilities are essential.
Location & Salary
Location: Survey No. 5, 6, 7A, Village Poicha (Rania), Taluka Savli, District Vadodara – 391780.
Salary: Competitive salary offered based on qualifications and experience.
Application Process
Interested candidates are encouraged to apply early to secure their position.
Email your CV to: vinayak@lactoseindialimited.com
Apply before November 10, 2025, to join one of India’s most trusted pharmaceutical companies!
Why Join Lactose India Limited?
- WHO–GMP certified state-of-the-art facility.
- Exposure to world-class manufacturing processes and technologies.
- Career advancement opportunities across departments.
- Work environment that promotes learning and innovation.
FAQs
1. Who can apply for Lactose India Limited openings?
Candidates with degrees in B.Pharm, M.Pharm, BSc, MSc, or Engineering (Mechanical, Chemical) are eligible. Freshers can apply for trainee positions.
2. Where is the company located?
The company is located in Poicha (Rania), Taluka Savli, Vadodara, Gujarat.
3. What is the selection process?
Shortlisted candidates will be contacted for a personal or virtual interview by the HR team.
4. Are freshers eligible?
Yes, freshers can apply for the Engineering Trainee role.
5. How to apply?
Send your updated resume to vinayak@lactoseindialimited.com.
Summary Table
| Company | Lactose India Limited |
| Vacancies | Multiple openings across 8 departments |
| Required Education | B.Pharm, M.Pharm, BSc, MSc, Diploma, BE Chemical/Mechanical |
| Experience | 0–5 Years (Freshers eligible for trainee role) |
To apply for this job email your details to vinayak@lactoseindialimited.com


