Ipca Laboratories Hiring API Production & QC

- API Production & QC Openings for UG/PG in Indore
- Company Overview
- Job Role & Responsibilities
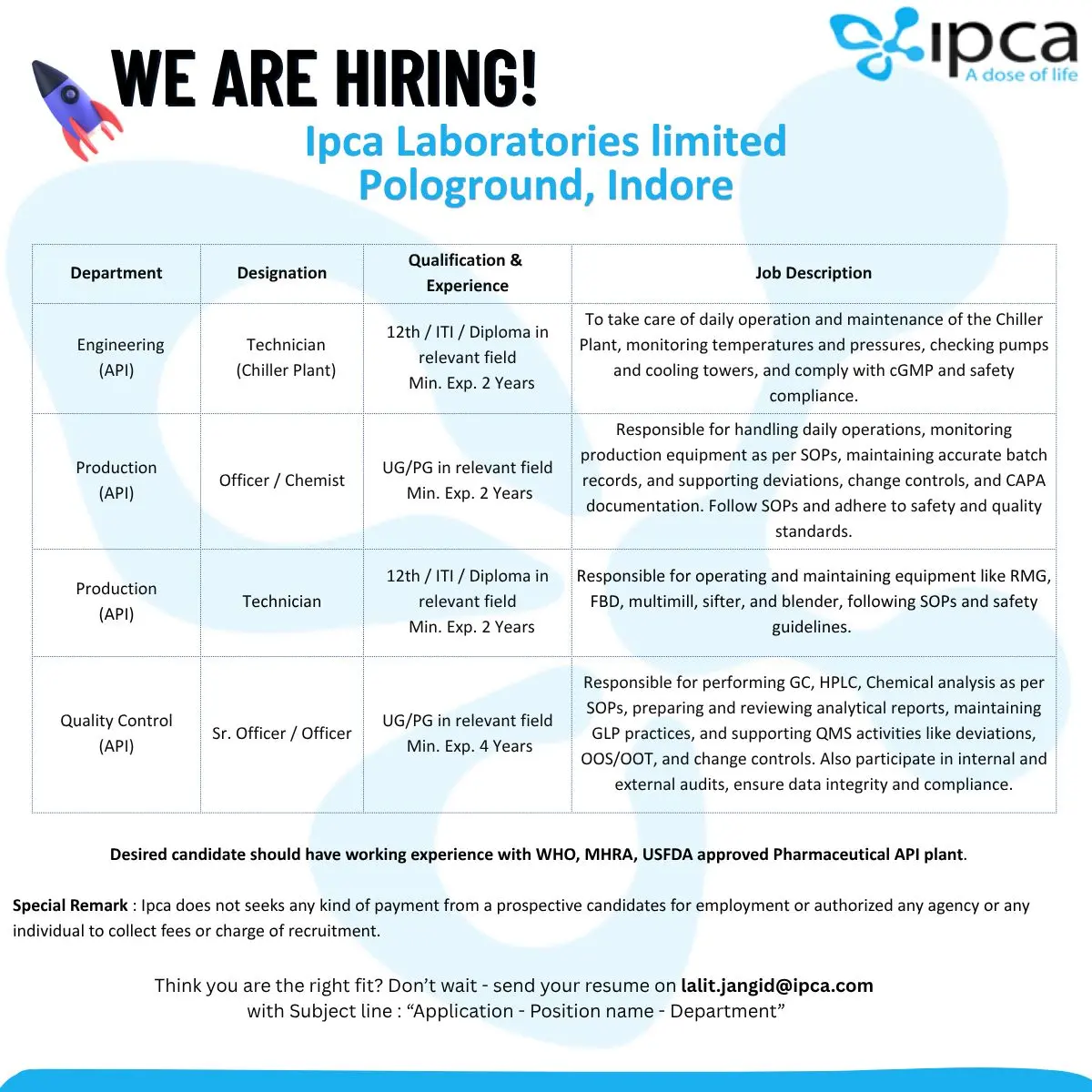
- Engineering (API) – Technician (Chiller Plant)
- Production (API) – Officer / Chemist
- Production (API) – Technician
- Quality Control (API) – Sr. Officer / Officer
- Eligibility / Qualifications
- Location & Salary
- Application Process
- FAQs
- Summary Table
API Production & QC Openings for UG/PG in Indore
Ipca Laboratories hiring UG/PG candidates with 2–4+ years experience for API Production, QC, and Engineering roles in Indore.
Ipca Laboratories is recruiting skilled professionals for its API manufacturing facility in Pologround, Indore. These openings span Production, Quality Control, and Engineering operations. Candidates with hands-on exposure to cGMP environments, validated equipment, and regulated market compliance will find strong long-term opportunities here. The company is looking for individuals who can work in fast-moving API operations and maintain quality, safety, and data integrity standards.
Company Overview
Ipca Laboratories is an established pharmaceutical manufacturer with a global footprint across more than 120 countries. Known for its strong API and formulation capabilities, the company operates with full regulatory compliance, supplying to WHO, MHRA, and USFDA-approved markets. Its Indore unit supports high-volume API manufacturing with modern infrastructure, controlled systems, and validated processes. This environment offers professionals the ability to enhance their technical skills in a structured, compliance-driven setup.
Job Role & Responsibilities
Openings are available in Engineering, Production, and Quality Control. Each role requires technical proficiency and strong adherence to SOPs.
Engineering (API) – Technician (Chiller Plant)
Qualification: 12th / ITI / Diploma in relevant field
Experience: Minimum 2 years
Responsibilities:
- Operating and maintaining chiller plant operations
- Monitoring temperature, pressure, and system parameters
- Inspecting pumps, cooling towers, and connected utilities
- Maintaining compliance with cGMP and safety standards
Production (API) – Officer / Chemist
Qualification: UG / PG in relevant field
Experience: Minimum 2 years
Responsibilities:
- Handling daily plant operations and equipment monitoring
- Maintaining batch records and documenting activities
- Supporting deviations, change controls, and CAPA workflows
- Ensuring adherence to SOPs, safety protocols, and quality standards
Production (API) – Technician
Qualification: 12th / ITI / Diploma in relevant field
Experience: Minimum 2 years
Responsibilities:
- Operating and managing RMG, FBD, multimill, sifter, blender
- Conducting routine checks and cleaning as per SOPs
- Ensuring equipment is used safely and efficiently
Quality Control (API) – Sr. Officer / Officer
Qualification: UG / PG in relevant field
Experience: Minimum 4 years
Responsibilities:
- Performing GC, HPLC, and chemical analysis
- Preparing and reviewing analytical documentation
- Maintaining GLP standards and data integrity
- Supporting QMS activities including OOS, OOT, deviations, and change controls
- Participating in internal and external audits
Preferred background: Experience in WHO, MHRA, or USFDA-approved API facilities.
Eligibility / Qualifications
Acceptable degrees and specializations include: Chemistry, Analytical Chemistry, Industrial Chemistry, Organic Chemistry, Pharmaceutical Chemistry, Biotechnology, Microbiology, Biochemistry, Life Sciences.
Location & Salary
Location: Ipca Laboratories Ltd., Pologround, Indore.
Salary: As per industry standards and experience.
Application Process
Candidates who match the criteria may apply by emailing their resume to: lalit.jangid@ipca.com
Subject Line: “Application – Position Name – Department”
Ipca clearly states it does not charge any fees or authorize any agency for recruitment.
FAQs
Is prior API experience required?
Yes. Most roles require 2–4+ years of hands-on API experience.
Are QC candidates required to know HPLC and GC?
Yes. These skills are essential for QC roles.
Can diploma holders apply?
Yes. Diploma and ITI candidates can apply for Technician roles.
Is regulatory exposure mandatory?
Experience in WHO, MHRA, USFDA-approved plants is preferred.
How should candidates apply?
Email your resume with the specified subject line.
Summary Table
| Category | Details |
|---|---|
| Company | Ipca Laboratories Ltd. |
| Vacancies | API Production, QC, and Engineering roles |
| Required Education | UG/PG, ITI, Diploma (Chemistry/Life Sciences) |
| Experience | 2–4+ years depending on role |
To apply for this job email your details to lalit.jangid@ipca.com


