Aurobindo Walk-in R&D & Analytical

- M.Pharm R&D & Analytical Openings at Aurobindo
- Company Overview
- Job Role & Responsibilities
- Formulation Research & Development (M.Pharm – Pharmaceutics)
- Formulation Analytical R&D – Method Development & Validation (M.Pharm / M.Sc)
- Eligibility / Qualifications
- Location & Salary
- Application Process
- FAQs
M.Pharm R&D & Analytical Openings at Aurobindo
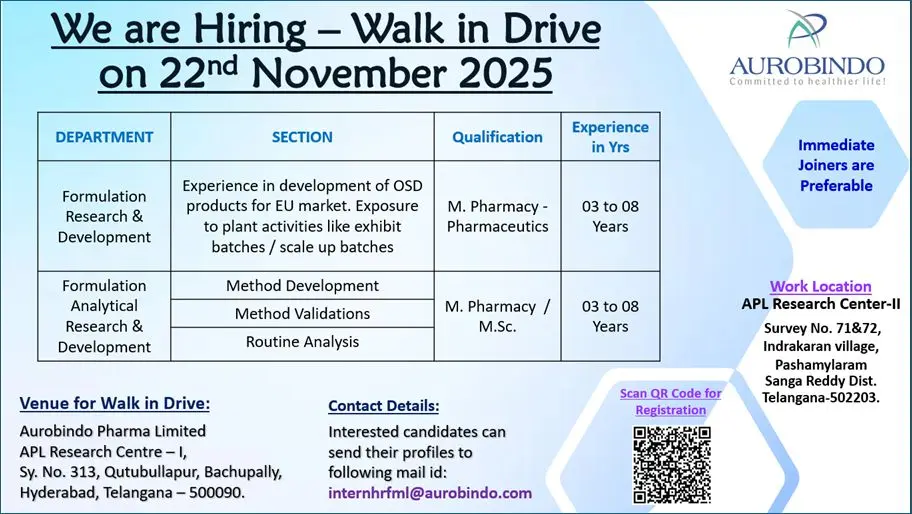
M.Pharm/M.Sc roles in Formulation & Analytical R&D (3–8 yrs) at Aurobindo Pharma Research Centre — Hyderabad. Walk-in 22 Nov.
Aurobindo Pharma is inviting experienced formulation and analytical R&D professionals to its research centres for a focused walk‑in hiring drive. These roles are ideal for M.Pharm and M.Sc candidates with 3–8 years’ experience in OSD product development for regulated markets, analytical method development and validation, and stability or scale‑up exposure. Immediate joiners who understand EU market requirements and GMP/GLP expectations will find this a strong, career‑defining opportunity.
Company Overview
Aurobindo Pharma is a leading integrated pharmaceutical company with global manufacturing and R&D capabilities. The company is recognized for its development of complex oral solid dosage (OSD) products, high‑volume manufacturing, and regulatory approvals across stringent markets. Aurobindo’s Research Centres focus on translational development — taking lab formulations to exhibit and scale‑up batches that meet EU and other regulated market standards. Joining Aurobindo means working within structured quality systems, validated processes, and cross‑functional teams that support product approvals and market access worldwide.
Job Role & Responsibilities
Aurobindo is recruiting for two related streams: Formulation Research & Development and Formulation Analytical R&D. Both streams require hands‑on technical skills, strong documentation habits, and experience with GMP environments.
Formulation Research & Development (M.Pharm – Pharmaceutics)
Experience: 3–8 years
Key responsibilities:
- Lead pre‑formulation and formulation development for OSD products targeted at EU markets.
- Design and execute lab, pilot, and exhibit/scale‑up batches; coordinate tech transfer to plant operations.
- Optimize formulation and process parameters to meet dissolution, stability and manufacturability targets.
- Prepare and maintain development records, batch reports and scale‑up documentation in line with GMP.
- Interact with analytical teams to define in‑process controls and release criteria.
- Support root‑cause analysis for formulation or stability issues and implement CAPA.
Ideal candidates will have proven success taking formulations from development to scale‑up, with awareness of regulatory expectations for EU dossiers.
Formulation Analytical R&D – Method Development & Validation (M.Pharm / M.Sc)
Experience: 3–8 years
Key responsibilities:
- Develop and validate analytical methods (HPLC, dissolution, assay, impurity, related substances) for OSD products.
- Execute routine analysis and support stability study testing.
- Prepare method validation protocols, reports and analytical procedures compliant with ICH and pharmacopeial standards.
- Troubleshoot analytical anomalies and support data integrity and GLP practices.
- Collaborate with formulation teams to ensure analytical methods support product specifications and regulatory dossiers.
This role demands strong hands‑on chromatography skills, method validation experience, and meticulous documentation.
Eligibility / Qualifications
Required qualifications:
- M.Pharm (Pharmaceutics) for Formulation R&D.
- M.Pharm or M.Sc (Analytical/Pharmaceutical Chemistry) for Analytical R&D.
Experience: 3 to 8 years in formulation development, analytical method development/validation, and stability testing for OSD products.
Technical proficiencies:
- HPLC method development and validation, dissolution testing, impurity profiling, stability study design.
- Experience with scale‑up/exhibit batches and tech transfer to manufacturing plants.
- Strong knowledge of ICH guidelines (Q2(R1), Q1A(R2)), pharmacopeial standards and EU dossier expectations.
- Familiarity with GMP, GLP, data integrity (ALCOA+), and laboratory quality systems.
Location & Salary
Work Location: APL Research Centre‑II, Survey No. 71 & 72, Indrakaran village, Pashamylaram, Sangareddy District, Telangana – 502203.
Walk‑in Venue for interview: Aurobindo Pharma Limited, APL Research Centre‑I, Sy. No. 313, Qutubullapur, Bachupally, Hyderabad, Telangana – 500090.
Application Process
Walk‑in Date: 22 November 2025
Time: (As per notification — candidates should scan the QR code for registration and slot details)
If you cannot attend the walk‑in or prefer to pre‑register, send your detailed CV and a one‑page summary of your development or analytical projects to: internhrfml@aurobindo.com. Use subject line: Application – Formulation/Analytical R&D – [Your Name].
Documents to carry for walk‑in:
- Updated CV with project details and clear role descriptions
- Original and photocopies of degree/mark sheets
- Experience letters and last 3 months’ payslips (if applicable)
- Government ID (Aadhaar/PAN)
FAQs
Q: What exact roles are open?
A: Formulation Research & Development (M.Pharm – Pharmaceutics) and Formulation Analytical R&D (Method Development & Validation) for M.Pharm/M.Sc candidates.
Q: What experience is required?
A: 3–8 years in relevant formulation development or analytical method development and validation.
Q: Is scale‑up or plant exposure mandatory?
A: Yes. Candidates should have exposure to exhibit or scale‑up batches and tech transfer activities to manufacturing plants, especially for EU‑targeted products.
Q: How do I register for the walk‑in?
A: Scan the QR code on the job notice for registration. You can also email your profile to internhrfml@aurobindo.com.
Q: Will remote interviews be available?
A: Aurobindo prefers walk‑in attendance for these technical roles but may consider virtual follow‑ups for shortlisted candidates.
Q: What should I include in my CV?
A: Highlight project responsibilities, methods you developed/validated, scale‑up batches handled, regulatory interactions (if any), and measurable outcomes (e.g., improved dissolution, reduced impurity levels).
| Category | Details |
|---|---|
| Company | Aurobindo Pharma Ltd. |
| Vacancies | Formulation R&D (M.Pharm), Formulation Analytical R&D (M.Pharm/M.Sc) |
| Required Education | M.Pharm (Pharmaceutics), M.Pharm/M.Sc (Analytical/Pharmaceutical Chemistry) |
| Experience | 3–8 years |