SynZeal Pharmachem Walk-in Production & QC

- B.Sc/M.Sc Production & QC Vacancies Ankleshwar
- Company Overview
- Job Role & Responsibilities
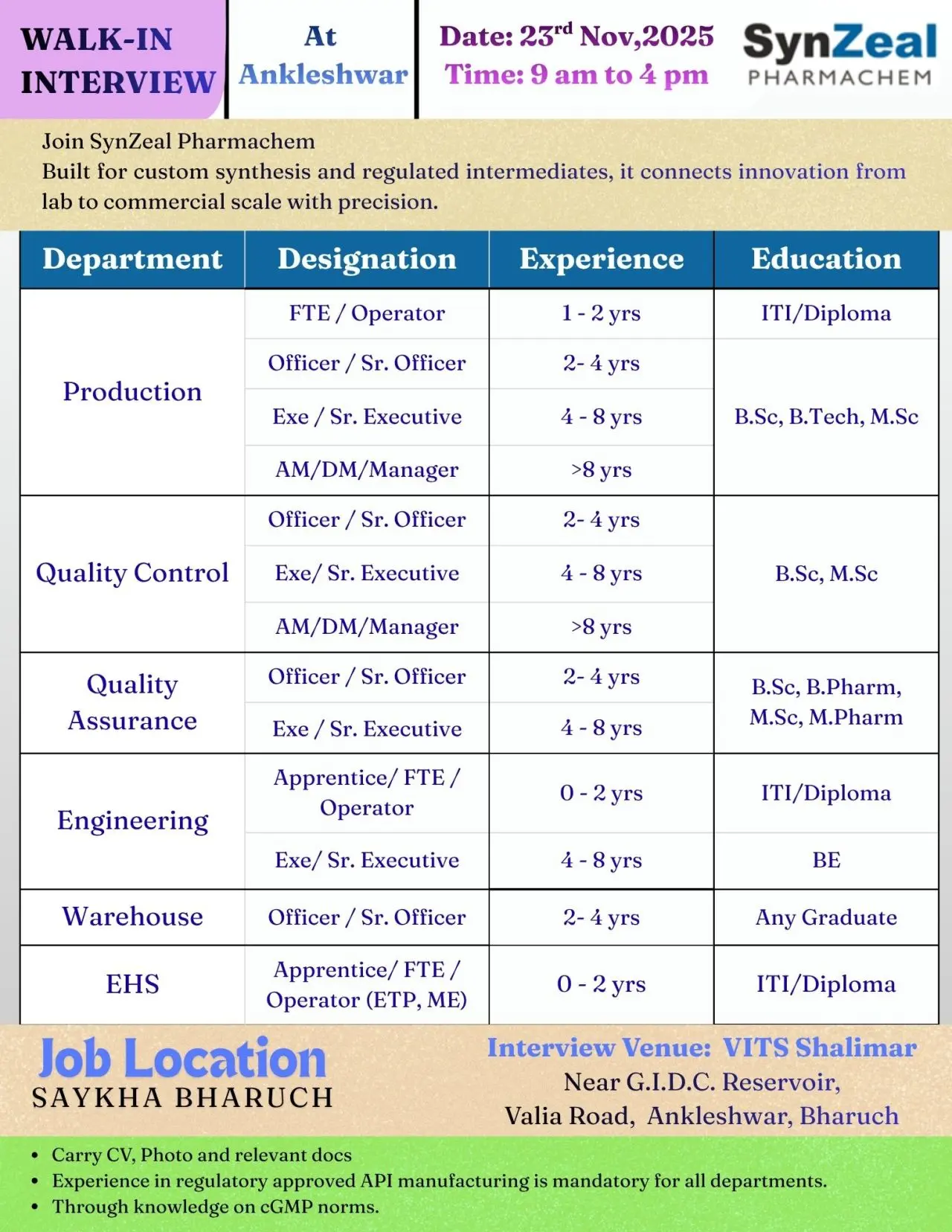
- Production
- Quality Control
- Quality Assurance
- Engineering
- Warehouse
- EHS
- Eligibility / Qualifications
- Location & Salary
- Application Process
- FAQs
- Summary Table
B.Sc/M.Sc Production & QC Vacancies Ankleshwar
SynZeal Pharmachem hiring B.Sc/M.Sc candidates for Production, QC, QA, Engineering, Warehouse and EHS in Ankleshwar. Walk-in on 23 Nov.
Intro Paragraph
SynZeal Pharmachem is conducting a large-scale walk-in drive for multiple departments at its regulated API manufacturing site. This opening is suited for candidates with hands-on experience in Production, Quality Control, Quality Assurance, Engineering, Warehouse and EHS, especially those who have worked in regulatory-approved API facilities and understand cGMP expectations.
Company Overview
SynZeal Pharmachem specializes in custom synthesis and regulated intermediates, supporting projects from laboratory development to commercial manufacturing. The organisation focuses on precision chemistry, compliance with international regulatory standards and robust scale-up capabilities. With strong experience across regulated markets, SynZeal offers a stable platform for technical and operational growth.
Job Role & Responsibilities
SynZeal is hiring across several functional areas with roles ranging from Apprentice/Operator level to Assistant Manager, Deputy Manager and Manager.
Production
- Operate equipment as per SOPs and maintain batch records.
- Support scale-up and manufacturing activities.
- Maintain process hygiene, safety compliance and cGMP documentation.
Quality Control
- Perform routine analysis using instruments such as HPLC, GC and wet chemistry.
- Review analytical reports and support OOS/OOT investigations.
- Ensure compliance to regulatory standards and data integrity.
Quality Assurance
- Manage QMS activities including change controls, deviations and CAPA.
- Conduct document review and support audit readiness.
- Ensure compliance to cGMP and regulatory norms.
Engineering
- Support utilities, maintenance and equipment readiness.
- Ensure timely calibration and upkeep of critical equipment.
- Maintain safety standards and support preventive maintenance.
Warehouse
- Manage RM/PM/FG handling, documentation and stock control.
- Ensure material traceability and cGMP-compliant storage.
EHS
- Support effluent treatment operations (ETP/MEP).
- Monitor safety compliance and execute routine inspections.
Eligibility / Qualifications
Education Requirements:
- ITI, Diploma, B.Sc, B.Pharm, B.Tech, BE, M.Sc, M.Pharm
Relevant Courses: Chemical Engineering, Industrial Chemistry, Pharmaceutical Chemistry, Microbiology, Biotechnology, QA & QC Techniques, Engineering Maintenance, EHS and Industrial Safety.
Experience:
- Apprentice/FTE/Operator: 0–2 years
- Officer/Sr. Officer: 2–4 years
- Executive/Sr. Executive: 4–8 years
- AM/DM/Manager: >8 years
Mandatory: Experience in regulatory-approved API manufacturing and strong knowledge of cGMP.
Location & Salary
Job Location: Saykha, Bharuch
Salary: As per company standards and experience level.
Application Process
Walk-in Interview Details:
Date: 23 November 2025
Time: 9:00 AM to 4:00 PM
Venue: VITS Shalimar, Near GIDC Reservoir, Valia Road, Ankleshwar, Bharuch
Candidates must carry:
- Updated CV
- Passport-size photo
- Educational and experience documents
For candidates unable to attend, contact details can be requested at the venue.
FAQs
Q: Is regulatory API experience mandatory?
Yes. Only candidates with experience in regulatory-compliant API plants will be considered.
Q: Which documents should I bring?
CV, photograph and all supporting educational/experience certificates.
Q: Are freshers eligible?
Freshers are eligible only for EHS and Engineering Apprentice/FTE roles.
Summary Table
| Category | Details |
|---|---|
| Company | SynZeal Pharmachem |
| Vacancies | Production, QC, QA, Engineering, Warehouse, EHS |
| Required Education | ITI, Diploma, B.Sc, B.Pharm, B.Tech, BE, M.Sc, M.Pharm |
| Experience | 0–>8 years depending on role |