Dishman Walk-In QA, QC, Production, R&D

- Company Overview
- Job Role & Responsibilities
- Quality Assurance – API & Formulation (Bavla)
- Quality Control – API (Naroda)
- Quality Control – AMV (Bavla)
- Production – Formulation (Softgel)
- Production – Packaging & Operators (Softgel)
- Research & Development Roles
- Eligibility / Qualifications
- Required Education
- Experience
- Location & Salary
- Walk-In Interview Details
- Application Process
- Why Build Your Career at Dishman Carbogen Amcis
- Frequently Asked Questions (FAQs)
- Who can attend this walk-in interview?
- Is this walk-in open for freshers?
- Can I apply if I cannot attend in person?
- Where will I be posted after selection?
- Summary Table
Dishman Walk-In – QA, QC, Production, R&D – Ahmedabad
Dishman Carbogen Amcis hiring QA, QC, Production & R&D professionals in Ahmedabad. 2–15 years experience. Walk-in 21 Dec 2025.
Dishman Carbogen Amcis has announced a large-scale walk-in interview drive at its Corporate House in Ahmedabad for experienced professionals across Quality Assurance, Quality Control, Production (Formulation – Softgel), and Research & Development functions. This hiring drive supports Dishman’s growing API and formulation operations at its Bavla and Naroda manufacturing plants.
The walk-in is designed for mid-level to senior pharmaceutical professionals with hands-on experience in regulated manufacturing, quality systems, analytical laboratories, and R&D process development. Candidates seeking long-term, compliance-driven careers in a global pharmaceutical organization will find this opportunity highly relevant.
Company Overview
Dishman Carbogen Amcis is a globally integrated pharmaceutical company specializing in API manufacturing, formulations, contract research, and development services. With a strong footprint across India, Europe, and global regulated markets, the company is recognized for its scientific depth, compliance culture, and technology-driven operations.
Dishman operates USFDA, EU-GMP compliant facilities and supports global innovators and generic pharmaceutical companies through end-to-end solutions, from research and development to commercial manufacturing. Careers at Dishman offer exposure to complex molecules, advanced analytical platforms, and international regulatory standards.
Job Role & Responsibilities
Multiple roles are open across Quality, Production, and R&D functions. Selected candidates will be responsible for ensuring product quality, regulatory compliance, and efficient manufacturing or research execution.
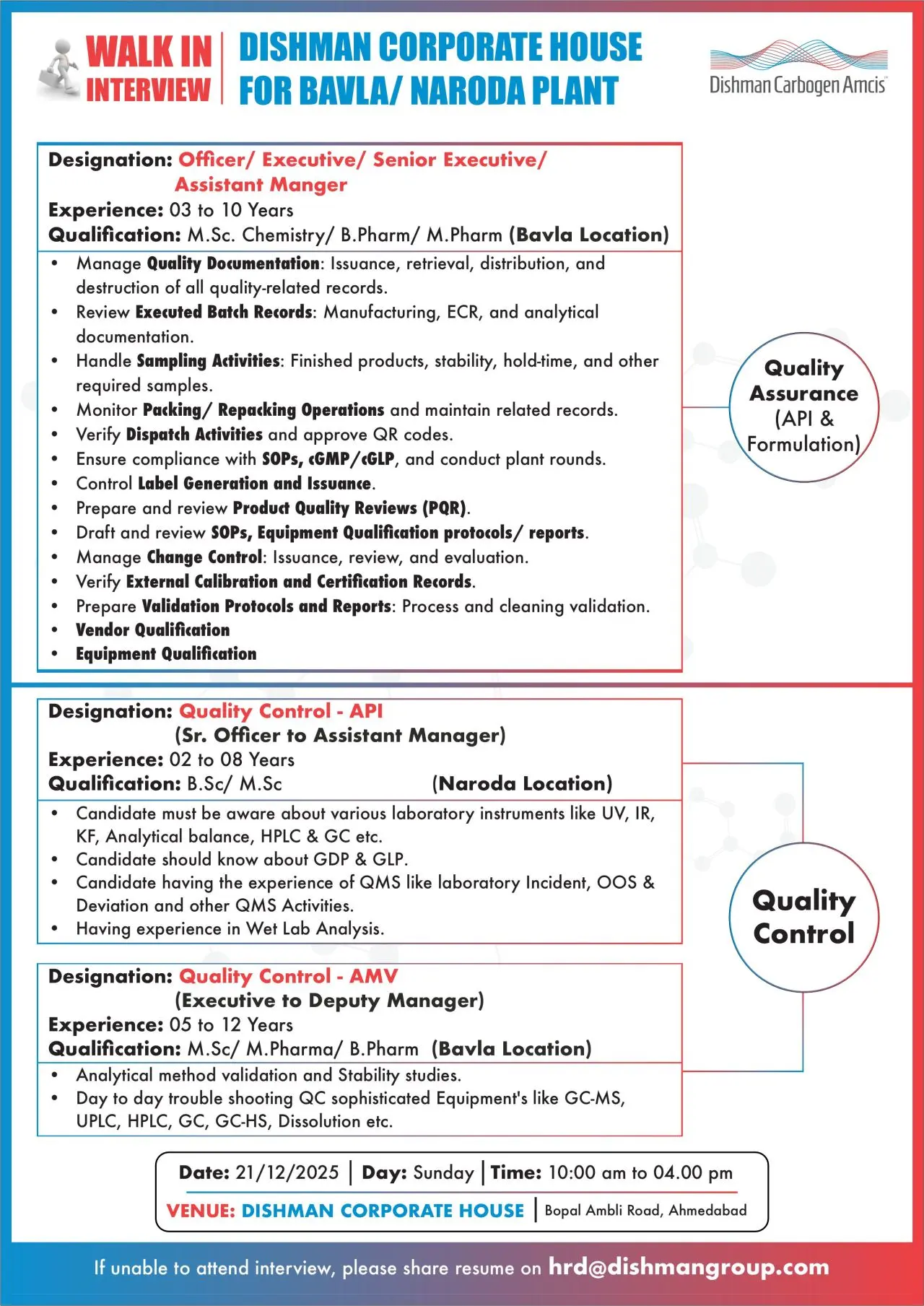
Quality Assurance – API & Formulation (Bavla)
- Manage quality documentation including issuance, retrieval, distribution, and archival
- Review executed batch manufacturing records, ECRs, and analytical documentation
- Handle sampling activities for finished products, stability, and hold-time samples
- Monitor packing and repacking operations and maintain compliance records
- Verify dispatch activities and approve QR codes
- Conduct plant rounds and ensure compliance with cGMP, GLP, and data integrity practices
- Prepare and review Product Quality Reviews (PQR)
- Draft and review SOPs, equipment qualification protocols, and reports
- Manage change control, deviations, and CAPA implementation
- Prepare and review validation protocols and reports (process and cleaning)
- Handle vendor qualification, equipment qualification, and external calibration records
Quality Control – API (Naroda)
- Perform wet lab analysis and routine analytical testing
- Operate and troubleshoot laboratory instruments including HPLC, GC, UV, IR, KF, and analytical balances
- Ensure compliance with GDP and GLP requirements
- Handle laboratory QMS activities such as OOS, deviations, and incident management
- Maintain accurate analytical documentation and laboratory records
Quality Control – AMV (Bavla)
- Perform analytical method validation and stability studies
- Troubleshoot sophisticated QC instruments such as HPLC, UPLC, GC, GC-MS, and dissolution systems
- Support day-to-day analytical operations and investigation activities
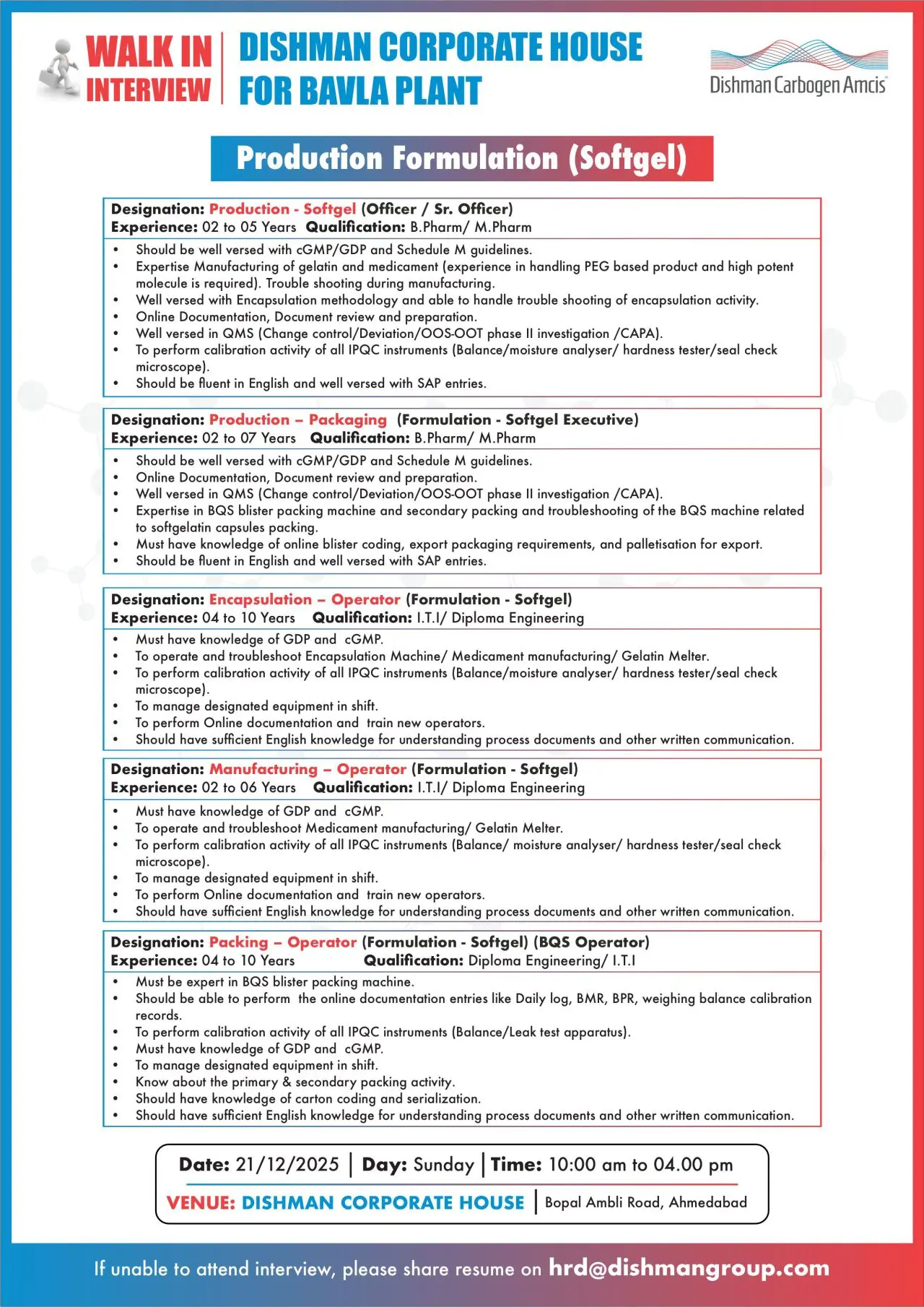
Production – Formulation (Softgel)
- Execute softgel manufacturing operations including gelatin and medicament preparation
- Handle PEG-based products and high-potency molecules
- Perform encapsulation operations and troubleshooting
- Manage online documentation, SAP entries, and batch record review
- Perform calibration of IPQC instruments
- Support QMS activities including change control, deviations, and CAPA
Production – Packaging & Operators (Softgel)
- Operate and troubleshoot BQS blister packing machines
- Handle primary and secondary packing activities
- Perform online documentation including BMR, BPR, and equipment logs
- Manage calibration of IPQC instruments and packing equipment
- Support serialization, carton coding, and export packaging requirements
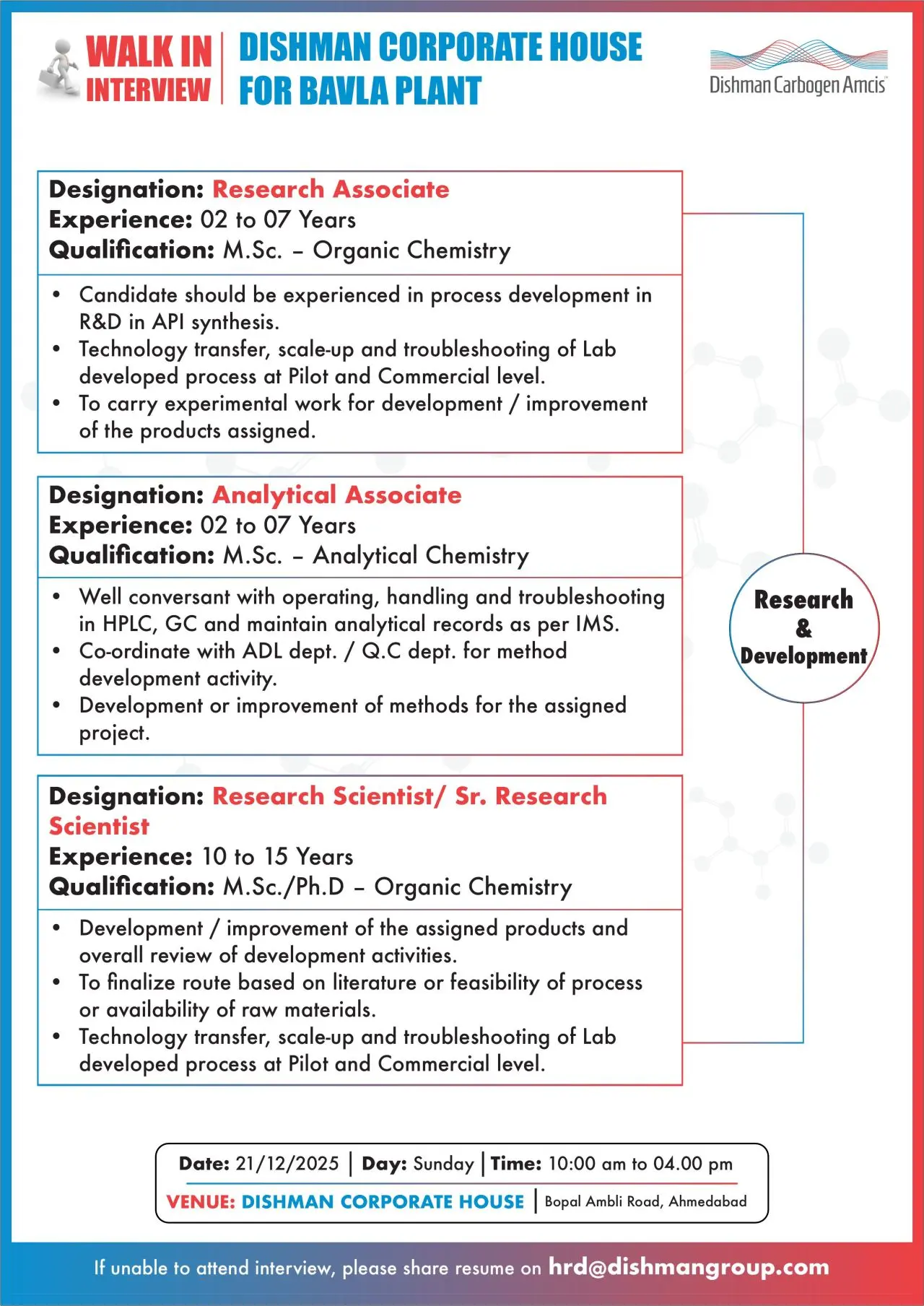
Research & Development Roles
Research Associate / Analytical Associate / Research Scientist
- Conduct process development for API synthesis
- Perform experimental work for product development and improvement
- Support technology transfer, scale-up, and troubleshooting at pilot and commercial levels
- Operate analytical instruments such as HPLC and GC for method development
- Finalize synthetic routes based on feasibility, literature, and raw material availability
Eligibility / Qualifications
Required Education
M.Sc. Chemistry, M.Sc. Organic Chemistry, M.Sc. Analytical Chemistry, B.Pharmacy, M.Pharmacy, B.Sc., Diploma Engineering, ITI
Experience
- Quality Assurance: 3–10 years
- Quality Control (API): 2–8 years
- Quality Control (AMV): 5–12 years
- Production (Softgel): 2–10 years
- R&D Roles: 2–15 years
Location & Salary
- Job Locations: Bavla Plant, Naroda Plant (Gujarat)
- Interview Venue: Dishman Corporate House, Bopal Ambli Road, Ahmedabad
- Salary: Competitive and aligned with industry standards, based on role and experience
Walk-In Interview Details
- Date: 21st December 2025 (Sunday)
- Time: 10:00 AM to 04:00 PM
- Venue:
Dishman Corporate House
Bopal Ambli Road, Ahmedabad
Application Process
Eligible candidates can directly attend the walk-in interview.
Candidates unable to attend may share their updated resume at:
Email: hrd@dishmangroup.com
Why Build Your Career at Dishman Carbogen Amcis
Dishman offers professionals the opportunity to work on complex APIs, advanced formulations, and high-end research projects within a globally regulated environment. Exposure to international clients, advanced technologies, and strong quality systems makes Dishman an ideal organization for long-term career growth in pharmaceuticals.
Frequently Asked Questions (FAQs)
Who can attend this walk-in interview?
Experienced professionals from Quality, Production, and R&D functions meeting the specified criteria can attend.
Is this walk-in open for freshers?
No. Most roles require prior industry experience.
Can I apply if I cannot attend in person?
Yes. Candidates may email their resumes to the HR contact provided.
Where will I be posted after selection?
Selected candidates will be placed at Bavla or Naroda plants based on role requirements.
Summary Table
| Company | Dishman Carbogen Amcis |
|---|---|
| Vacancies | Multiple |
| Required Education | M.Sc. Chemistry, M.Sc. Organic Chemistry, M.Sc. Analytical Chemistry, B.Pharm, M.Pharm, Diploma Engineering, ITI |
| Experience | 2–15 years (Role-dependent) |