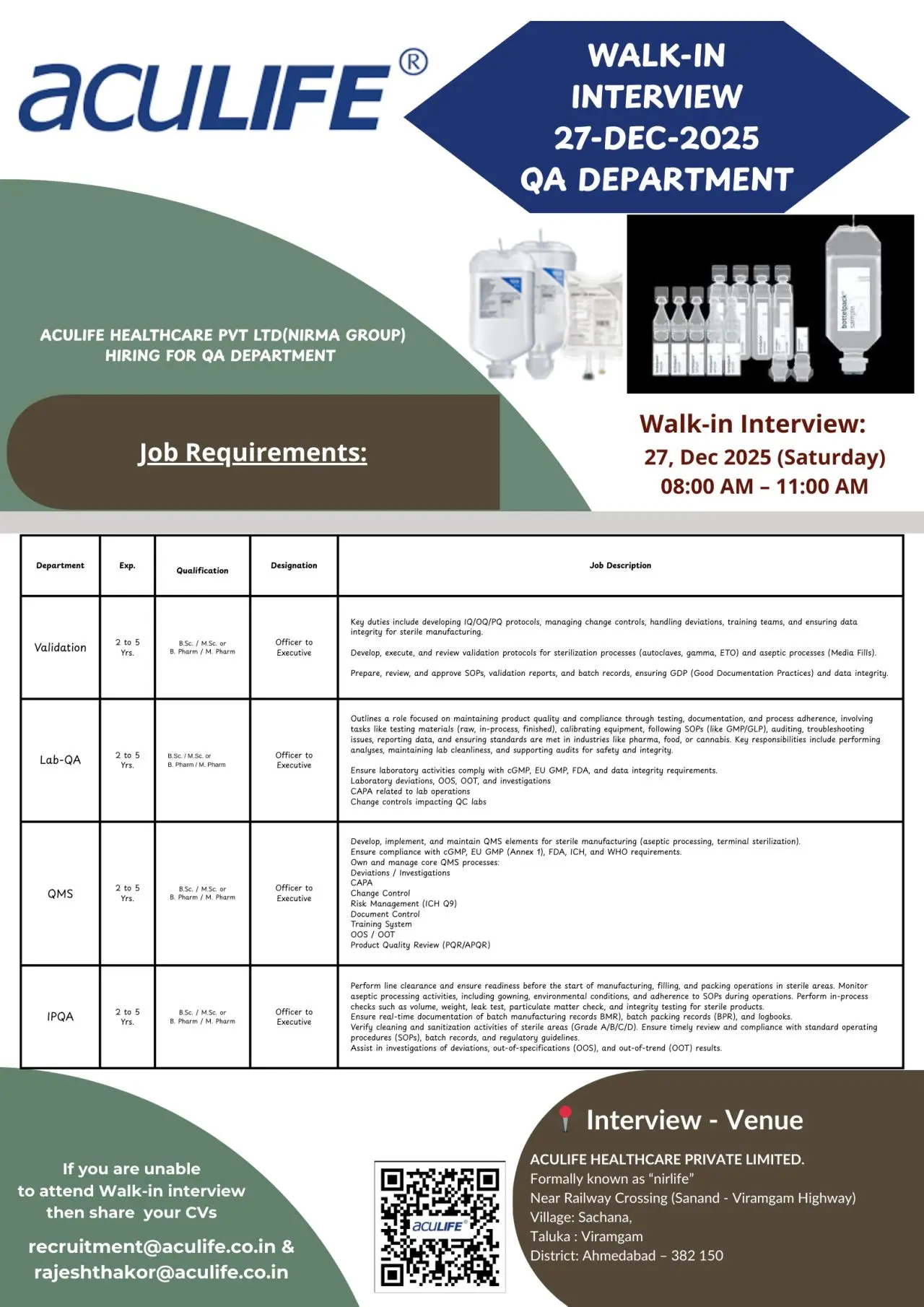
Aculife walk-in QA Officers & Executives

- Company Overview
- Job Role & Responsibilities
- Validation – QA (Officer to Executive)
- Lab QA / QMS
- QMS – Sterile Manufacturing
- IPQA – Sterile Operations
- Eligibility / Qualifications
- Location & Salary
- Walk-in Interview Details
- Application Process
- FAQs
QA Officer Executive Jobs – BSc BPharm – Ahmedabad | Aculife
Aculife Healthcare hiring QA Officers & Executives with 2–5 years experience. BSc, MSc, BPharm eligible. Walk-in on 27 Dec 2025, Ahmedabad.
Aculife Healthcare Pvt. Ltd., a part of the Nirma Group, is conducting a walk-in interview for experienced Quality Assurance professionals at its sterile manufacturing facility near Ahmedabad. This opportunity is ideal for candidates seeking regulated pharma QA jobs in validation, QMS, lab QA, and IPQA within a globally compliant sterile manufacturing environment. Professionals with hands-on exposure to cGMP, EU GMP, and FDA-regulated operations will find strong long-term career growth and stability at Aculife.
Company Overview
Aculife Healthcare Pvt. Ltd., formerly known as Nirlife, operates under the Nirma Group and is a well-established pharmaceutical manufacturer with a strong presence in sterile formulations and injectables. The company follows stringent global regulatory standards including cGMP, EU GMP, FDA, WHO, and ICH guidelines. Aculife is known for its robust quality systems, data integrity culture, and continuous compliance-driven operations.
Job Role & Responsibilities
Validation – QA (Officer to Executive)
- Prepare, execute, and review IQ, OQ, and PQ protocols for equipment and processes
- Validate sterilization processes including autoclaves, gamma irradiation, and ETO
- Manage media fills and aseptic process validations
- Handle change controls, deviations, and CAPA related to validation activities
- Ensure data integrity and GDP compliance for sterile manufacturing
Lab QA / QMS
- Ensure laboratory compliance with cGMP, EU GMP, FDA, and data integrity requirements
- Review laboratory deviations, OOS, OOT, and investigation reports
- Manage CAPA and change controls impacting QC laboratories
- Support internal, external, and regulatory audits
QMS – Sterile Manufacturing
- Develop and maintain QMS elements for aseptic and terminally sterilized products
- Own core quality processes including deviations, investigations, CAPA, and risk management (ICH Q9)
- Manage document control, training systems, and APQR/PQR activities
IPQA – Sterile Operations
- Perform line clearance and ensure readiness for manufacturing, filling, and packing
- Monitor aseptic operations including gowning, environmental conditions, and SOP adherence
- Conduct in-process checks such as volume, weight, leak tests, and integrity testing
- Review BMRs, BPRs, and logbooks in real time
- Support investigations for deviations, OOS, and OOT
Eligibility / Qualifications
- Education: B.Sc., M.Sc., B.Pharm, M.Pharm
- Experience: 2 to 5 years in sterile pharmaceutical manufacturing
- Strong knowledge of cGMP, EU GMP (Annex 1), FDA, ICH, WHO guidelines
- Experience in validation, QMS, lab QA, or IPQA roles is mandatory
Location & Salary
- Location: Sachana, Viramgam Taluka, Ahmedabad District, Gujarat
- Salary: Best in industry, commensurate with experience and skill set
Walk-in Interview Details
- Date: 27 December 2025 (Saturday)
- Time: 08:00 AM to 11:00 AM
- Venue: Aculife Healthcare Pvt. Ltd. (formerly Nirlife), Near Railway Crossing, Sanand–Viramgam Highway, Village Sachana, Taluka Viramgam, District Ahmedabad – 382150
Application Process
Candidates unable to attend the walk-in may share their updated CVs via email:
FAQs
Who can attend this walk-in interview?
Candidates with 2–5 years of QA experience in sterile pharmaceutical manufacturing.
Is freshers eligible for this role?
No. This hiring is strictly for experienced professionals.
What regulatory exposure is required?
Hands-on exposure to cGMP, EU GMP, FDA, and data integrity practices is mandatory.
Is this role suitable for injectable manufacturing experience?
Yes. Sterile and injectable manufacturing experience is strongly preferred.
| Company | Aculife Healthcare Pvt. Ltd. (Nirma Group) |
|---|---|
| Vacancies | Multiple (QA Validation, QMS, Lab QA, IPQA) |
| Required Education | B.Sc., M.Sc., B.Pharm, M.Pharm |
| Experience | 2–5 Years |