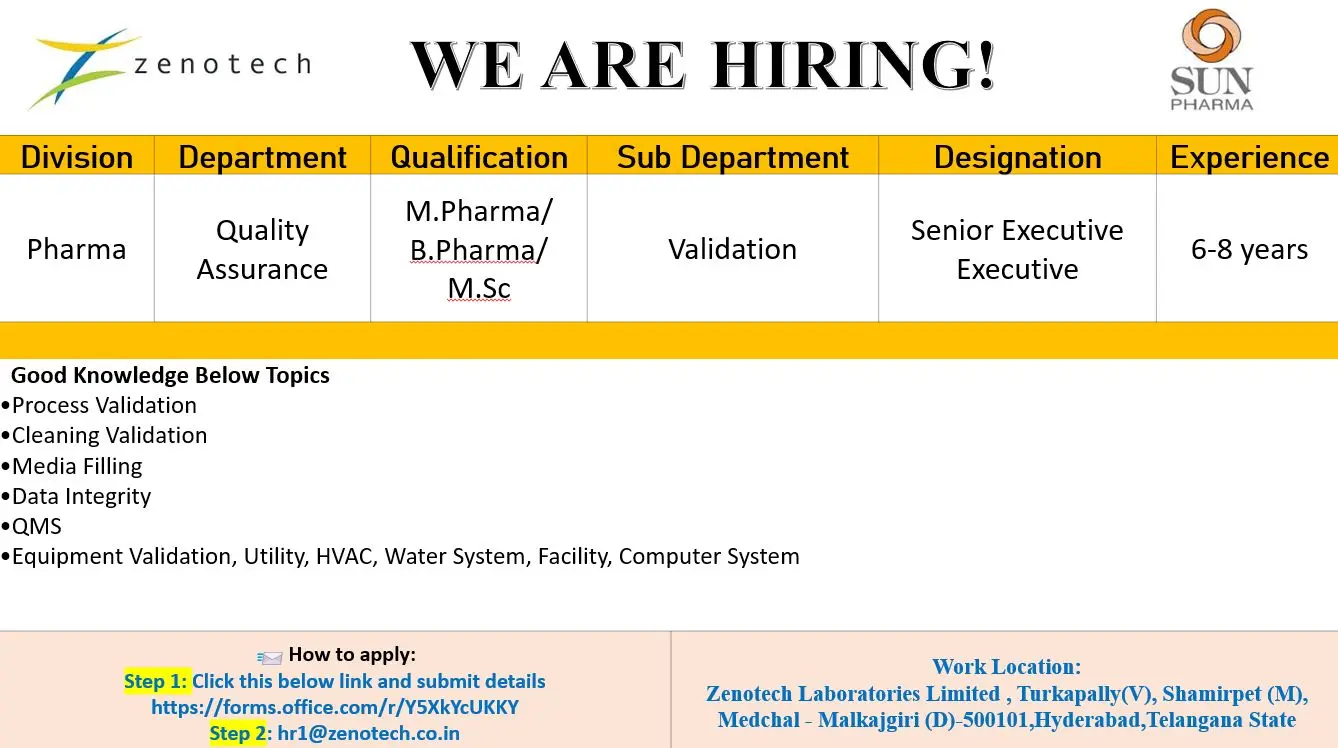
Sun Pharma Hiring QA Senior Executive

- Company Overview
- Job Role & Responsibilities
- Key Responsibilities
- Eligibility / Qualifications
- Educational Background
- Experience Requirement
- Location & Salary
- Application Process
- Why Join Zenotech Laboratories?
- FAQs
M.Pharm/B.Pharm QA Executive Jobs | 1 Vacancy | Hyderabad
M.Pharm, B.Pharm, M.Sc QA Validation Executive vacancy at Zenotech, Hyderabad. 6–8 years experience. Apply now.
Zenotech Laboratories Limited, a Sun Pharma company, is expanding its Quality Assurance division at its Hyderabad facility and invites experienced regulatory and validation professionals to join a highly regulated pharmaceutical manufacturing environment. This opportunity is ideal for candidates seeking senior-level pharma QA validation jobs with strong exposure to global GMP, data integrity systems, and lifecycle quality management within a reputed formulation and API manufacturing organization.
Company Overview
Zenotech Laboratories Limited operates as part of the Sun Pharmaceutical Industries ecosystem and is recognized for its robust manufacturing practices, regulatory compliance, and commitment to high-quality pharmaceutical products. The organization supports regulated markets and maintains compliance with international guidelines including USFDA, WHO-GMP, and global data integrity standards. Working at Zenotech provides long-term career stability, advanced validation exposure, and the opportunity to contribute directly to patient safety and healthcare excellence.
Job Role & Responsibilities
As a Senior Executive / Executive – Quality Assurance (Validation), the selected professional will play a critical role in ensuring validated processes, systems, and facilities comply with regulatory expectations and internal quality systems.
Key Responsibilities
- Execute and review process validation, cleaning validation, and media fill studies in accordance with approved protocols
- Ensure compliance with data integrity principles and ALCOA+ guidelines across validation activities
- Handle equipment qualification including IQ, OQ, and PQ for manufacturing and utility systems
- Validate and maintain HVAC, water systems, utilities, facilities, and computerized systems
- Support and manage QMS activities including deviations, CAPA, change control, and risk assessments
- Prepare, review, and approve validation protocols, reports, and SOPs
- Support internal audits, regulatory inspections, and validation-related observations
- Collaborate with production, engineering, QC, and IT teams to ensure end-to-end validation compliance
Eligibility / Qualifications
Educational Background
M.Pharm, B.Pharm, M.Sc (Pharmaceutical Sciences, Quality Assurance, Pharmaceutical Analysis, Industrial Pharmacy, Life Sciences)
Experience Requirement
- 6 to 8 years of relevant experience in pharmaceutical Quality Assurance – Validation
- Proven exposure to regulated manufacturing environments
- Strong working knowledge of validation lifecycle management and regulatory expectations
Location & Salary
- Work Location: Zenotech Laboratories Limited, Turkapally Village, Shamirpet Mandal, Medchal–Malkajgiri District, Hyderabad, Telangana
- Salary: Best in industry, commensurate with experience and expertise
Application Process
Interested candidates can apply using either of the following methods:
- Step 1: Submit your details through the official application link:
https://forms.office.com/r/Y5XkYcUKKY - Step 2: Email your updated resume to hr1@zenotech.co.in
Ensure your resume highlights hands-on validation experience and regulatory exposure.
Why Join Zenotech Laboratories?
- Work with a Sun Pharma–backed organization
- Exposure to high-value validation and compliance projects
- Strong regulatory learning and career growth potential
- Stable work environment with global quality standards
FAQs
Q1: Who can apply for this QA Validation role?
Candidates with M.Pharm, B.Pharm, or M.Sc degrees and 6–8 years of QA validation experience in pharma can apply.
Q2: Is regulatory audit exposure required?
Yes. Experience handling audits, inspections, and validation-related compliance is highly preferred.
Q3: What validation areas will I work on?
Process validation, cleaning validation, media fill, equipment qualification, HVAC, water systems, utilities, and computerized systems.
Q4: Where is the job location?
The position is based at Zenotech Laboratories Limited, Turkapally, Hyderabad.
| Company | Zenotech Laboratories Limited (Sun Pharma Group) |
|---|---|
| Vacancies | 1 |
| Required Education | M.Pharm, B.Pharm, M.Sc |
| Experience | 6–8 Years |
You must sign in to apply for this position.


