Neuland Hiring Production, QA, QC, Engineering & Safety

- Company Overview
- Job Role & Responsibilities
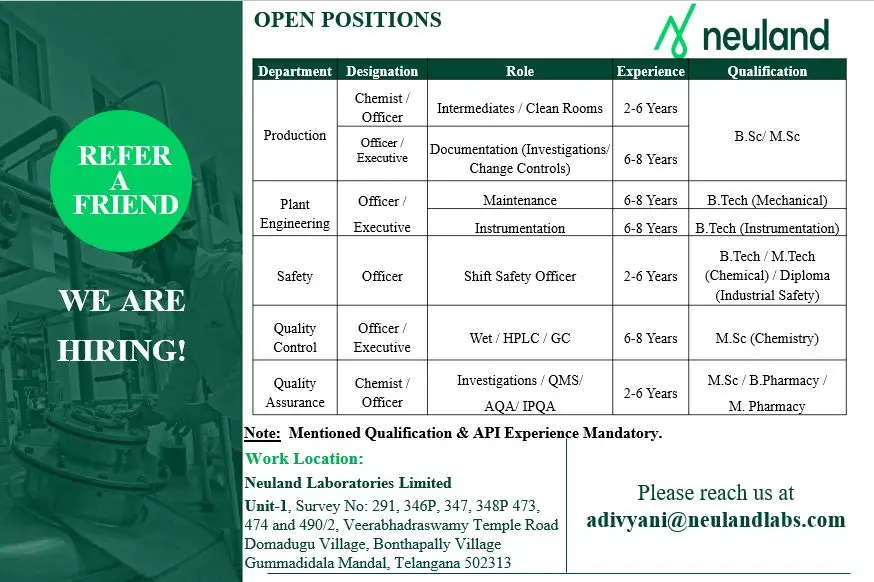
- Production – Chemist / Officer / Executive (API)
- Plant Engineering – Officer / Executive
- Safety – Shift Safety Officer
- Quality Control – Officer / Executive
- Quality Assurance – Chemist / Officer
- Eligibility / Qualifications
- Educational Qualification
- Experience Requirement
- Location & Salary
- Application Process
- FAQs
- Who can apply for these roles?
- Is API experience mandatory?
- Are freshers eligible?
- What qualifications are accepted?
- Where is the work location?
- Summary Table
B.Sc, M.Sc, B.Pharm Jobs – Neuland Labs Telangana
Neuland Laboratories hiring Production, QA, QC, Engineering & Safety roles in Telangana. API experience mandatory. 2–8 years exp.
Neuland Laboratories Limited is inviting applications from experienced pharmaceutical professionals for multiple openings across Production, Quality Assurance, Quality Control, Plant Engineering, and Safety departments at its API manufacturing facility in Telangana. This hiring drive targets candidates with hands-on exposure to regulated active pharmaceutical ingredient (API) manufacturing, clean room operations, and compliance-driven quality systems.
These opportunities are ideal for professionals searching for API pharma jobs in Telangana, production chemist roles, QA QMS jobs, QC HPLC analyst positions, plant engineering maintenance jobs, and industrial safety officer roles in pharmaceutical manufacturing plants. Neuland Laboratories offers a technically advanced work environment, strong compliance culture, and long-term career growth in the API segment.
Company Overview
Neuland Laboratories Limited is a globally recognized API manufacturing company with a strong presence in regulated international markets. The company specializes in complex active pharmaceutical ingredients and follows stringent quality, safety, and environmental standards across its manufacturing operations.
With state-of-the-art facilities and a strong focus on regulatory compliance, Neuland Laboratories operates under global GMP guidelines and international audit expectations. Working at Neuland provides professionals exposure to advanced chemical processes, robust quality systems, and continuous improvement initiatives within the pharmaceutical API industry.
Job Role & Responsibilities
Production – Chemist / Officer / Executive (API)
Production professionals will be responsible for day-to-day API manufacturing activities.
Key responsibilities include:
- Handling intermediates and clean room operations
- Executing production documentation, investigations, and change controls
- Ensuring compliance with GMP and safety procedures
- Coordinating with QA, QC, and engineering teams
Plant Engineering – Officer / Executive
Engineering professionals will manage maintenance and instrumentation activities.
Responsibilities include:
- Preventive and breakdown maintenance of plant equipment
- Instrumentation calibration and compliance
- Supporting utility operations and equipment qualification
- Ensuring audit readiness and documentation compliance
Safety – Shift Safety Officer
Safety officers will oversee EHS activities across manufacturing shifts.
Responsibilities include:
- Implementing industrial safety practices
- Monitoring compliance with safety regulations
- Conducting safety audits and risk assessments
- Training employees on safety procedures
Quality Control – Officer / Executive
QC professionals will perform analytical testing and quality evaluation.
Responsibilities include:
- Wet chemical analysis and instrumental analysis using HPLC and GC
- Testing of raw materials, intermediates, and finished API products
- Maintaining GLP-compliant documentation
- Supporting investigations and regulatory audits
Quality Assurance – Chemist / Officer
QA professionals will manage quality systems and compliance functions.
Responsibilities include:
- Handling investigations, change controls, and QMS activities
- Analytical QA and in-process quality assurance (IPQA)
- Ensuring compliance with SOPs, GMP, and regulatory guidelines
- Supporting internal and external audits
Eligibility / Qualifications
Educational Qualification
B.Sc Chemistry, M.Sc Chemistry, B.Pharm, M.Pharm, B.Tech Mechanical, B.Tech Instrumentation, M.Tech Chemical, Diploma in Industrial Safety
Experience Requirement
- Production: 2 to 6 years
- Plant Engineering: 6 to 8 years
- Safety: 2 to 6 years
- Quality Control: 6 to 8 years
- Quality Assurance: 2 to 6 years
Note: Relevant API manufacturing experience is mandatory for all positions.
Location & Salary
The work location is Neuland Laboratories Limited, Unit-1, Gummadidala Mandal, Telangana. Salary will be competitive and aligned with industry standards based on role, qualification, and experience. Final compensation details will be discussed during the interview process.
Application Process
Interested and eligible candidates should share their updated CV to:
Candidates are advised to clearly mention the applied position and department in the email subject line.
FAQs
Who can apply for these roles?
Candidates with relevant API manufacturing experience can apply.
Is API experience mandatory?
Yes. API experience is mandatory for all listed positions.
Are freshers eligible?
No. These roles are open to experienced professionals only.
What qualifications are accepted?
Science, pharmacy, engineering, and industrial safety qualifications as specified are eligible.
Where is the work location?
The work location is Neuland Laboratories Limited, Unit-1, Telangana.
Summary Table
| Company | Neuland Laboratories Limited |
|---|---|
| Vacancies | Production, QA, QC, Engineering, Safety |
| Required Education | B.Sc, M.Sc, B.Pharm, M.Pharm, B.Tech, M.Tech, Diploma |
| Experience | 2–8 Years |
You must sign in to apply for this position.


