BDR Pharmaceuticals Walk-in Warehouse, QC, QA, Production

- Company Overview
- Job Role & Responsibilities
- Quality Control (QC)
- Quality Assurance (QA)
- Warehouse & QMS
- Production & Packing (Injectables / OSD)
- Engineering & Utilities
- Eligibility / Qualifications
- Educational Background
- Experience Range
- Key Requirements
- Location & Salary
- Walk-in Interview Details
- Application Process
- FAQs
- Who should attend this walk-in interview?
- Is USFDA experience mandatory?
- Are freshers eligible?
- Can I apply online instead of attending the walk-in?
- Summary Table
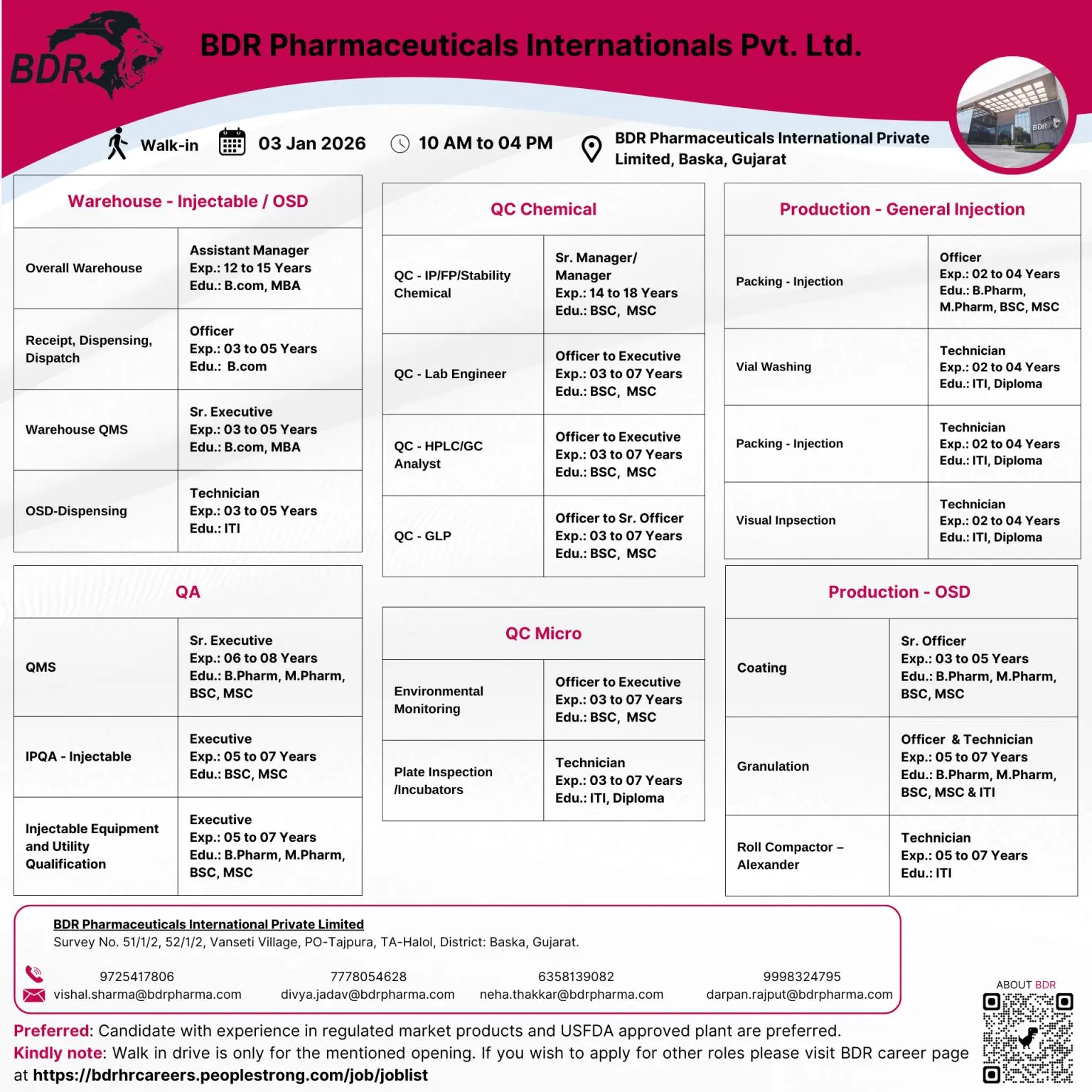
BDR Pharmaceuticals International Pvt. Ltd. is conducting a large-scale walk-in interview drive at its regulated manufacturing facility in Baska, Gujarat, inviting experienced pharmaceutical professionals across Quality Control, Quality Assurance, Warehouse, Production, Packing, and Injectable operations. This hiring drive is focused on strengthening teams supporting USFDA-regulated injectable and OSD manufacturing, offering long-term career growth in a compliance-driven environment.
This opportunity is ideal for candidates searching for pharma jobs in Gujarat, QC QA jobs in injectable plants, warehouse roles in pharmaceutical companies, and production careers in USFDA-approved facilities. The walk-in is scheduled for 03 January 2026, with multiple roles open across senior management, executive, officer, and technician levels.
Company Overview
BDR Pharmaceuticals International Pvt. Ltd. is a globally active pharmaceutical company with a strong presence in regulated markets. The organization specializes in complex generics, injectables, oncology, and specialty pharmaceutical products. With a robust compliance framework aligned to USFDA, EU-GMP, and other international regulatory standards, BDR has built a reputation for quality, reliability, and scientific excellence.
The Baska manufacturing facility in Gujarat is a critical hub for injectable and OSD operations, supported by advanced quality systems, modern infrastructure, and experienced technical leadership. Working at BDR offers exposure to high-compliance manufacturing, regulated documentation systems, and continuous professional development.
Job Role & Responsibilities
BDR Pharmaceuticals is hiring across multiple departments. Key responsibilities vary by role and department but broadly include:
Quality Control (QC)
- Chemical analysis of IP, FP, stability, and raw materials
- Operation of HPLC, GC, and other analytical instruments
- GLP compliance, data integrity, and documentation
- Environmental monitoring and microbiological coordination
- Instrument qualification and troubleshooting
Quality Assurance (QA)
- IPQA activities for injectable and OSD operations
- QMS documentation, deviations, CAPA, and change controls
- Batch record review and compliance verification
- Support for regulatory audits and inspections
Warehouse & QMS
- Receipt, dispensing, storage, and dispatch of materials
- SAP documentation and inventory control
- Warehouse QMS compliance and audit readiness
- Handling RM, PM, FG, and injectable components
Production & Packing (Injectables / OSD)
- Injectable filling, vial washing, visual inspection
- OSD granulation, coating, roll compactor operations
- Equipment operation and basic troubleshooting
- Adherence to GMP, SOPs, and safety protocols
Engineering & Utilities
- Injectable equipment qualification
- Utility system handling and validation support
- Preventive maintenance coordination
Eligibility / Qualifications
Educational Background
B.Pharm, M.Pharm, B.Sc, M.Sc (Chemistry), B.Com, MBA, ITI, Diploma (relevant trades)
Experience Range
- Technician / Officer roles: 2 to 7 years
- Executive / Senior Executive roles: 3 to 8 years
- Manager / Assistant Manager roles: 12 to 18 years
Key Requirements
- Experience in injectable or OSD manufacturing preferred
- Exposure to USFDA or other regulated market facilities
- Strong documentation and compliance mindset
- Willingness to work in a regulated, shift-based environment
Location & Salary
Job location is BDR Pharmaceuticals International Pvt. Ltd., Baska (Halol Taluka), Gujarat. Salary and designation will be offered based on experience, role fitment, and interview performance, aligned with industry standards for regulated pharma plants.
Walk-in Interview Details
- Date: 03 January 2026
- Time: 10:00 AM to 04:00 PM
- Venue:
BDR Pharmaceuticals International Pvt. Ltd.
Survey No. 51/1/2, 52/1/2,
Vanseti Village, PO – Tajpura,
TA – Halol, District – Baska, Gujarat
Application Process
Candidates may attend the walk-in directly for the listed openings. Those unable to attend may apply online via the official career portal:
https://bdrhrcareers.peoplestrong.com/job/joblist
For queries, candidates may contact or email:
- vishal.sharma@bdrpharma.com | 7778054628
- divya.jadav@bdrpharma.com | 6358139082
- neha.thakkar@bdrpharma.com | 9998324795
- darpan.rajput@bdrpharma.com | 9725417806
FAQs
Who should attend this walk-in interview?
Experienced professionals from QC, QA, Production, Warehouse, Engineering, and Packing backgrounds, especially those with injectable or regulated plant exposure.
Is USFDA experience mandatory?
USFDA or regulated market experience is strongly preferred and will be an added advantage.
Are freshers eligible?
This walk-in is primarily for experienced candidates. Freshers should apply through the career portal for suitable openings.
Can I apply online instead of attending the walk-in?
Yes. Candidates may apply through the official BDR career portal if unable to attend in person.
Summary Table
| Company | BDR Pharmaceuticals International Pvt. Ltd. |
|---|---|
| Vacancies | QC, QA, Production, Packing, Warehouse, Engineering, Injectable Roles |
| Required Education | B.Pharm, M.Pharm, B.Sc, M.Sc, B.Com, MBA, ITI, Diploma |
| Experience | 2 to 18 years depending on role |