Zenotech Hiring QA Executive and QC Officer

- Company Overview
- Job Role & Responsibilities
- Quality Assurance – Executive / Senior Executive (6–8 Years)
- Quality Control – Officer / Senior Officer (3–5 Years)
- Eligibility / Qualifications
- Required Education
- Experience Requirement
- Location & Salary
- Application Process
- Why This Role Matters in Pharma Manufacturing
- Frequently Asked Questions (FAQs)
- Who can apply for these roles?
- Is injectable experience mandatory?
- What quality systems are followed at Zenotech?
- Where is the work location?
- Summary Table
QA & QC Jobs – B.Pharm/M.Pharm – Hyderabad
Zenotech hiring QA Executive and QC Officer in Hyderabad. B.Pharm, M.Pharm, MSc eligible. 3–8 years experience.
Zenotech Laboratories Limited, a part of the Sun Pharma ecosystem, is hiring experienced professionals for its Quality Assurance (QA) and Quality Control (QC) departments at its Hyderabad manufacturing facility. This opportunity is ideal for pharmaceutical professionals with solid exposure to injectable manufacturing, analytical testing, and GMP-regulated quality systems who are looking to grow their careers in a reputed formulation-focused organization.
These roles directly support product quality, regulatory compliance, and patient safety, making them critical positions within Zenotech’s pharmaceutical operations.
Company Overview
Zenotech Laboratories Limited is a well-established pharmaceutical company operating under the Sun Pharma group umbrella. The company specializes in formulation development and manufacturing, with a strong presence in regulated markets.
Zenotech is known for its focus on quality-driven manufacturing, compliance with global regulatory standards, and continuous improvement across quality systems. Its Hyderabad facility operates under stringent cGMP, GDP, and regulatory audit frameworks, supporting both domestic and international pharmaceutical supply chains.
Working at Zenotech offers professionals exposure to large-scale pharmaceutical operations, strong quality governance, and long-term career stability within the Indian pharma industry.
Job Role & Responsibilities
Zenotech is currently hiring for the following positions:
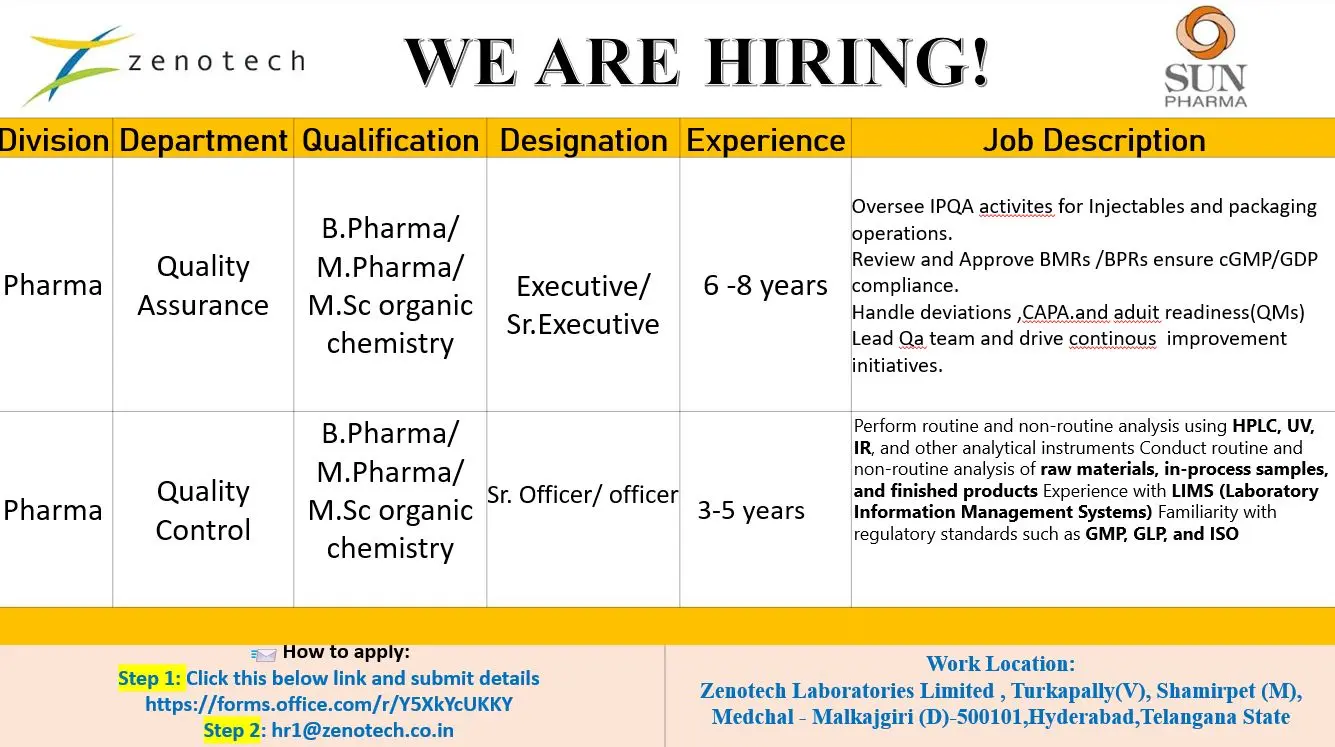
Quality Assurance – Executive / Senior Executive (6–8 Years)
- Oversee In-Process Quality Assurance (IPQA) activities for injectable manufacturing and packaging operations.
- Review and approve Batch Manufacturing Records (BMRs) and Batch Packaging Records (BPRs).
- Ensure compliance with cGMP, GDP, and internal quality standards.
- Handle deviations, change controls, and CAPA implementation.
- Support regulatory inspections and maintain continuous audit readiness.
- Lead QA teams and drive continuous improvement initiatives within quality systems.
Quality Control – Officer / Senior Officer (3–5 Years)
- Perform routine and non-routine analysis of raw materials, in-process samples, and finished products.
- Operate analytical instruments such as HPLC, UV, IR, and other laboratory equipment.
- Ensure accurate documentation and data integrity in laboratory records.
- Work with LIMS (Laboratory Information Management Systems) for sample tracking and data management.
- Follow regulatory standards including GMP, GLP, and ISO requirements.
These roles are essential for maintaining consistent product quality and regulatory compliance across injectable manufacturing operations.
Eligibility / Qualifications
Required Education
Candidates must possess one of the following qualifications:
B.Pharm, M.Pharm, M.Sc (Organic Chemistry)
Relevant Courses (comma-separated):
Pharmacy, Pharmaceutical Sciences, Organic Chemistry, Analytical Chemistry, Quality Assurance, Quality Control
Experience Requirement
- QA Executive / Sr. Executive: 6 to 8 years of relevant experience
- QC Officer / Sr. Officer: 3 to 5 years of relevant experience
Experience in injectable formulations and regulated pharmaceutical environments is strongly preferred.
Location & Salary
Job Location: Hyderabad, Telangana, India
Zenotech Laboratories Limited, Turkapally Village, Shamirpet Mandal, Medchal–Malkajgiri District – 500101
Salary: Competitive and aligned with industry standards, based on role, experience, and technical expertise.
Application Process
Interested candidates should follow the two-step application process below:
Step 1: Submit your details using the online form
https://forms.office.com/r/Y5XkYcUKKY
Step 2: Share your updated CV via email
Email: hr1@zenotech.co.in
Candidates are advised to mention the applied department (QA or QC) clearly in the subject line.
Why This Role Matters in Pharma Manufacturing
Quality Assurance and Quality Control play a vital role in ensuring the safety, efficacy, and consistency of pharmaceutical products, especially in injectable manufacturing. These positions directly support regulatory approvals, audit success, and patient safety outcomes.
Joining Zenotech allows professionals to work in a compliance-focused environment while contributing to high-quality pharmaceutical manufacturing aligned with global standards.
Frequently Asked Questions (FAQs)
Who can apply for these roles?
Candidates with B.Pharm, M.Pharm, or MSc (Organic Chemistry) qualifications and relevant experience can apply.
Is injectable experience mandatory?
Injectable experience is strongly preferred, especially for Quality Assurance roles.
What quality systems are followed at Zenotech?
Zenotech follows cGMP, GDP, GLP, ISO, and global regulatory compliance standards.
Where is the work location?
The roles are based at Zenotech Laboratories Limited, Hyderabad, Telangana.
Summary Table
| Company | Zenotech Laboratories Limited |
|---|---|
| Vacancies | Quality Assurance, Quality Control |
| Required Education | B.Pharm, M.Pharm, MSc Organic Chemistry |
| Experience | 3–8 years |
To apply for this job please visit forms.office.com.


