Stallion Hiring Production, QC, QA, Engineering

- Company Overview
- Job Role & Responsibilities
- Production – Operator / FTE
- Quality Control – Officer / Senior Officer
- Quality Assurance – Officer / Senior Officer
- Engineering – Officer / Senior Officer
- Eligibility / Qualifications
- Educational Background
- Experience Requirements
- Location & Salary
- Application Process
- Why Build a Career in Pharmaceutical Manufacturing
- Frequently Asked Questions (FAQs)
Pharma Jobs (BSc/MPharm, 4 Vacancies) – Production, QC, QA
Hiring Production, QC, QA, Engineering roles. 4 vacancies for BSc, MPharm, Diploma candidates with 2–5 years experience.
A leading pharmaceutical manufacturing organization is hiring experienced professionals across Production, Quality Control, Quality Assurance, and Engineering functions. These roles are ideal for candidates with hands-on exposure to solid oral dosage manufacturing, analytical quality control, validation, and plant engineering operations within a regulated pharmaceutical environment. If you are looking to advance your career in pharma manufacturing with strong compliance exposure, these opportunities offer stability, learning, and long-term growth.
Company Overview
The organization operates as a GMP-compliant pharmaceutical manufacturing unit supporting domestic and international markets. With a focus on quality, regulatory compliance, and operational excellence, the company maintains robust systems across production, quality, and engineering functions.
The facility follows current Good Manufacturing Practices (cGMP), quality management systems, and regulatory expectations to ensure safe, effective, and high-quality medicines. Employees are encouraged to follow structured processes, continuous improvement initiatives, and cross-functional collaboration to support healthcare outcomes.
Job Role & Responsibilities
Multiple departments are hiring professionals with 2–5 years of relevant pharmaceutical industry experience.
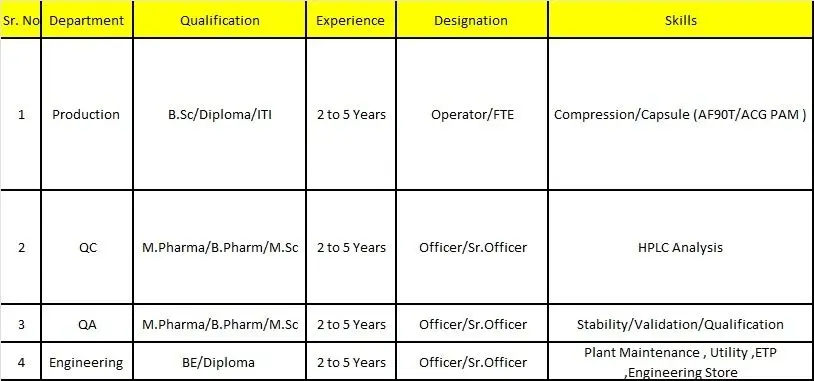
Production – Operator / FTE
Key responsibilities include:
- Operation of compression and capsule filling machines
- Handling of equipment such as AF90T and ACG PAM capsule machines
- Monitoring in-process parameters and recording batch data
- Ensuring adherence to GMP and safety requirements
- Supporting line clearance, equipment cleaning, and routine maintenance
Quality Control – Officer / Senior Officer
Key responsibilities include:
- Performing routine and non-routine analysis using HPLC
- Testing of raw materials, in-process samples, and finished products
- Documentation of analytical data and review of test results
- Compliance with GLP, SOPs, and regulatory requirements
- Supporting analytical investigations and stability testing
Quality Assurance – Officer / Senior Officer
Key responsibilities include:
- Execution and review of stability studies
- Participation in validation and qualification activities
- Review of batch manufacturing and packaging records
- Support for deviation handling, CAPA, and change control
- Ensuring compliance with GMP, SOPs, and audit requirements
Engineering – Officer / Senior Officer
Key responsibilities include:
- Plant maintenance for production and utility equipment
- Handling of utilities such as HVAC, water systems, and ETP
- Preventive and breakdown maintenance activities
- Coordination with engineering stores and spare management
- Documentation related to maintenance and engineering compliance
Eligibility / Qualifications
Educational Background
Candidates must meet one of the following qualification requirements based on the role:
Production:
B.Sc, Diploma, ITI
Quality Control / Quality Assurance:
B.Pharm, M.Pharm, M.Sc
Engineering:
B.E, Diploma (Mechanical / Electrical)
Relevant courses include:
B.Sc Chemistry, B.Pharm, M.Pharm, M.Sc Chemistry, Diploma in Mechanical Engineering, Diploma in Electrical Engineering, ITI
Experience Requirements
- 2 to 5 years of relevant experience in pharmaceutical manufacturing, quality, or engineering roles
- Prior experience in regulated GMP environments is mandatory
Location & Salary
- Job Location: As per manufacturing facility requirements
- Employment Type: Full-time
- Salary: Competitive and aligned with industry standards, based on role and experience
Application Process
Interested candidates can apply by sharing their updated resume through the official recruitment channel of the organization. Shortlisted candidates will be contacted for technical interviews and further evaluation.
Why Build a Career in Pharmaceutical Manufacturing
These roles offer direct exposure to regulated pharmaceutical operations, quality systems, and manufacturing technologies. Employees gain hands-on experience with GMP compliance, regulatory audits, and cross-functional collaboration, supporting long-term career progression in the pharmaceutical industry.
Frequently Asked Questions (FAQs)
Are these roles suitable for candidates with capsule and compression experience?
Yes. Production roles specifically require experience in compression and capsule operations.
Is HPLC experience mandatory for QC roles?
Yes. Hands-on HPLC analysis experience is required for Quality Control positions.
Do QA roles involve validation activities?
Yes. Stability studies, validation, and qualification activities are core responsibilities.
Is prior GMP exposure required?
Yes. All roles require experience in GMP-regulated pharmaceutical environments.
| Company | Stallion Laboratories Pvt. Ltd |
|---|---|
| Vacancies | 4 |
| Required Education | B.Sc, B.Pharm, M.Pharm, M.Sc, Diploma, ITI |
| Experience | 2–5 years |