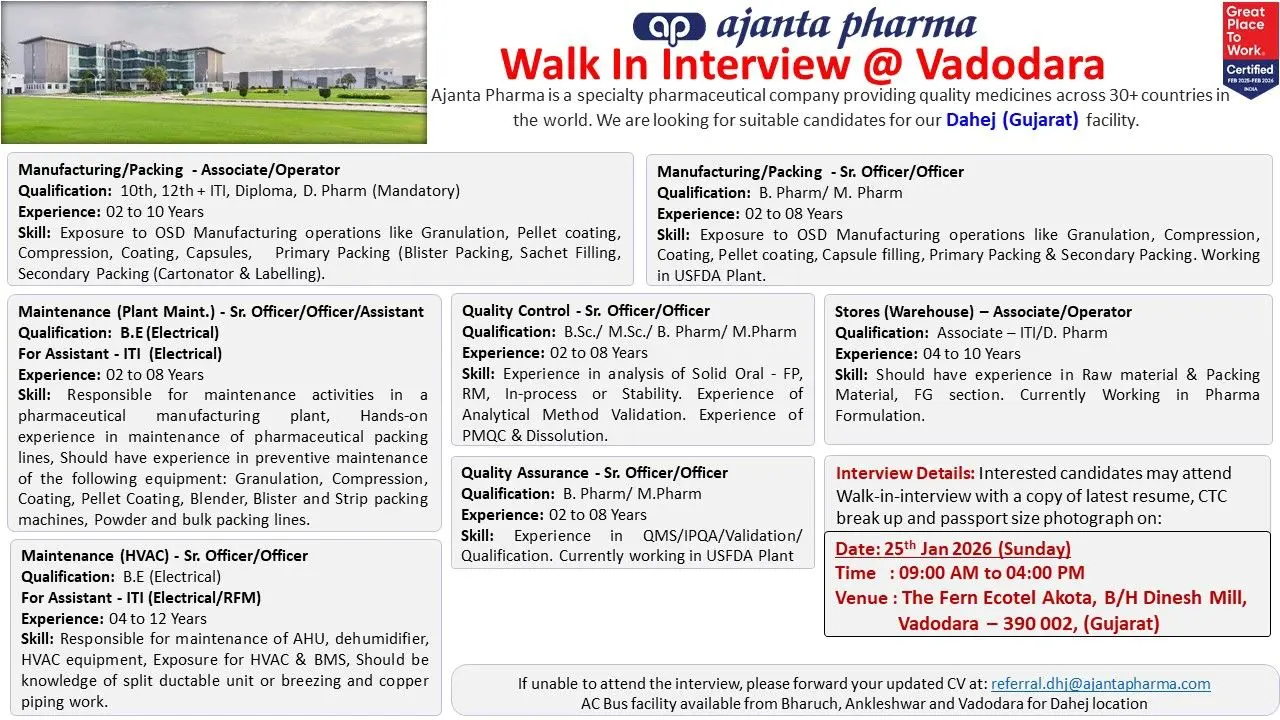
Ajanta Walk-in Manufacturing, QC, QA, Maintenance

- Company Overview
- Job Role & Responsibilities
- Manufacturing / Packing – Associate / Operator
- Manufacturing / Packing – Sr. Officer / Officer
- Quality Control – Sr. Officer / Officer
- Quality Assurance – Sr. Officer / Officer
- Maintenance – Plant Maintenance
- Maintenance – HVAC
- Stores / Warehouse – Associate / Operator
- Eligibility / Qualifications
- Location & Salary
- Walk-In Interview Details
- Ahmedabad Walk-In
- Vadodara Walk-In
- Application Process
- Why Join Ajanta Pharma
- Career Growth & Industry Exposure
- FAQs
- Who can attend the Ajanta Pharma walk-in interviews?
- Are freshers eligible?
- What manufacturing experience is preferred?
- Is transport provided for Dahej location?
- Can I apply if I cannot attend the walk-in?
- Job Summary Table
Pharma Vacancies B.Pharm/M.Pharm – Ajanta Dahej
Ajanta Pharma hiring Manufacturing, QC, QA, Maintenance roles. B.Pharm, M.Pharm, ITI candidates. Walk-in Feb 2026, Gujarat.
Ajanta Pharma is conducting large-scale walk-in interviews at Ahmedabad and Vadodara to recruit skilled professionals for its Dahej manufacturing facility in Gujarat. This hiring drive covers a wide range of roles across manufacturing, packing, quality control, quality assurance, maintenance, HVAC, engineering, and warehouse operations. Candidates with experience in regulated pharmaceutical plants, especially USFDA-approved facilities, will find strong long-term career opportunities with a globally respected pharmaceutical organization.
Company Overview
Ajanta Pharma is a specialty pharmaceutical company with a strong international footprint, supplying high-quality medicines to more than 30 countries worldwide. Known for its focus on branded generics and regulated markets, Ajanta Pharma operates modern manufacturing facilities that comply with global regulatory standards.
The company is certified as a Great Place to Work and is recognized for its employee-focused culture, strong compliance framework, and emphasis on quality-driven manufacturing. Ajanta’s Dahej facility plays a critical role in solid oral dosage (OSD) manufacturing and supports exports to highly regulated markets, including the United States.
Working at Ajanta Pharma offers professionals exposure to USFDA-compliant operations, advanced manufacturing technologies, and structured quality systems.
Job Role & Responsibilities
Ajanta Pharma is hiring for multiple departments at its Dahej plant. Responsibilities vary by role but are aligned with GMP-compliant pharmaceutical manufacturing and quality operations.
Manufacturing / Packing – Associate / Operator
- Executing OSD manufacturing activities such as granulation, pellet coating, compression, coating, and capsule filling
- Operating primary and secondary packing lines including blister packing, sachet filling, cartoning, and labelling
- Following SOPs, GMP guidelines, and safety standards
- Maintaining batch records and production documentation
Manufacturing / Packing – Sr. Officer / Officer
- Supervising OSD manufacturing and packing operations
- Ensuring compliance with USFDA and GMP requirements
- Coordinating with QA and QC teams during production activities
- Monitoring equipment performance and process controls
Quality Control – Sr. Officer / Officer
- Performing analysis of solid oral finished products, raw materials, in-process samples, and stability samples
- Conducting analytical method validation and dissolution testing
- Handling PMQC activities and laboratory documentation
- Supporting audits and regulatory inspections
Quality Assurance – Sr. Officer / Officer
- Managing QMS documentation, IPQA activities, and validation support
- Reviewing batch manufacturing and packing records
- Handling deviation management, CAPA, and qualification activities
- Working in USFDA-regulated plant environments
Maintenance – Plant Maintenance
- Executing preventive and breakdown maintenance activities
- Maintaining pharmaceutical manufacturing and packing equipment
- Handling granulation, compression, coating, pellet coating, blender, blister, strip, and bulk packing machines
- Ensuring equipment reliability and compliance
Maintenance – HVAC
- Managing AHU, dehumidifiers, and HVAC systems
- Supporting BMS operations and environmental control
- Handling ducting, copper piping, and HVAC maintenance activities
Stores / Warehouse – Associate / Operator
- Managing raw materials, packing materials, and finished goods
- Handling warehouse documentation and inventory control
- Ensuring GMP compliance in storage and material movement
Eligibility / Qualifications
Candidates must meet role-specific education and experience requirements.
Required Education (Role-Based):
- B.Pharm, M.Pharm
- B.Sc, M.Sc
- D.Pharm
- Diploma (Engineering / Pharmacy)
- ITI (Electrical, Mechanical, RFM)
- 10th, 12th
- B.E / B.Tech (Electrical)
Relevant Courses Accepted:
B.Pharmacy, M.Pharmacy, B.Sc Chemistry, B.Sc Microbiology, M.Sc Chemistry, M.Sc Microbiology, Diploma in Pharmacy, Diploma in Engineering, ITI Electrical, ITI Mechanical, ITI RFM, B.E Electrical
Experience Requirements:
- Manufacturing / Packing: 2–10 years
- QC / QA: 2–8 years
- Maintenance (Plant & HVAC): 2–12 years
- Stores / Warehouse: 4–10 years
Candidates with experience in solid oral dosage manufacturing will be preferred. Experience limited to injectables or APIs will not be considered for OSD-focused QC and QA roles.
Location & Salary
- Work Location: Dahej, Gujarat
- Interview Locations: Ahmedabad and Vadodara
- Salary: Best in industry and commensurate with experience
Ajanta Pharma also provides AC bus facilities from Bharuch, Ankleshwar, and Vadodara for employees working at the Dahej plant.
Walk-In Interview Details
Ahmedabad Walk-In
- Date: 1st February 2026 (Sunday)
- Time: 09:00 AM to 04:00 PM
- Venue:
Hotel Novotel,
Iscon Cross Road,
Sarkhej–Gandhinagar Highway,
Ahmedabad, Gujarat – 380015
Vadodara Walk-In
- Date: 25th January 2026 (Sunday)
- Time: 09:00 AM to 04:00 PM
- Venue:
The Fern Ecotel Akota,
Behind Dinesh Mill,
Vadodara – 390002, Gujarat
Candidates should carry:
- Updated resume
- CTC break-up details
- Passport-size photograph
Application Process
Candidates unable to attend the walk-in interviews may apply by sharing their updated CV via email.
Email ID:
referral.dhj@ajantapharma.com
Shortlisting will be based on relevant experience and role suitability.
Why Join Ajanta Pharma
- Great Place to Work certified organization
- Exposure to USFDA-compliant manufacturing
- Strong focus on quality, compliance, and employee development
- Stable long-term career opportunities
- Employee transport facilities for Dahej location
Career Growth & Industry Exposure
Ajanta Pharma provides professionals with hands-on exposure to regulated pharmaceutical manufacturing, global audits, and advanced OSD technologies. Employees gain strong technical, regulatory, and leadership skills, supporting long-term growth across manufacturing, quality, and engineering functions.
These roles directly support the supply of high-quality medicines to patients across global markets.
FAQs
Who can attend the Ajanta Pharma walk-in interviews?
Candidates with relevant pharma manufacturing, QC, QA, maintenance, or warehouse experience can attend.
Are freshers eligible?
Freshers are eligible only for selected roles where mentioned. Most positions require prior experience.
What manufacturing experience is preferred?
Solid oral dosage manufacturing experience is mandatory for most roles.
Is transport provided for Dahej location?
Yes. AC bus facilities are available from Bharuch, Ankleshwar, and Vadodara.
Can I apply if I cannot attend the walk-in?
Yes. You can email your CV to referral.dhj@ajantapharma.com.
Job Summary Table
Company Ajanta Pharma
Vacancies Multiple
Required Education B.Pharm, M.Pharm, B.Sc, M.Sc, D.Pharm, ITI, Diploma, B.E
Experience 2–12 Years