Hikal walk-in QC Officers & API Production

- Company Overview
- Job Role & Responsibilities
- Quality Control Officer – API
- API Production – Junior Officer / Officer
- Eligibility / Qualifications
- Required Education
- Experience Requirements
- Desired Skills
- Location & Salary
- Why Build a Career at Hikal Limited
- Application Process
- SEO-Focused Job Highlights
- Frequently Asked Questions (FAQs)
- Is this walk-in interview open for freshers?
- What qualifications are required for QC roles?
- Are female candidates eligible for API Production roles?
- Where is the walk-in interview location?
- Can I apply by email?
- Career Growth in API Manufacturing and Quality Control
- Summary Table
MSc Chemistry QC & API Production Jobs in Bangalore
Hikal Limited hiring QC Officers & API Production roles in Bangalore. MSc Chemistry, BSc eligible. 1–5 years experience required.
Hikal Limited is conducting a multi-day walk-in interview at its Bangalore manufacturing facility for Quality Control and API Production professionals. This hiring drive is aimed at strengthening Hikal’s regulated API manufacturing operations and quality systems. Candidates with hands-on experience in API manufacturing and analytical quality control are invited to attend and build long-term careers with a globally recognized pharmaceutical and specialty chemical company.
These openings offer exposure to regulated manufacturing practices, advanced analytical laboratories, and compliance-driven environments that directly support global healthcare supply chains.
Company Overview
Hikal Limited is a well-established global supplier of active pharmaceutical ingredients (APIs), intermediates, and specialty chemicals serving regulated markets across the US, Europe, and other international regions. The company operates multiple USFDA and international regulatory-approved manufacturing facilities in India and follows stringent quality, safety, and environmental standards.
Hikal’s API manufacturing and quality control operations play a critical role in supplying life-saving medicines to global pharmaceutical customers. The company is known for its strong compliance culture, robust analytical capabilities, and commitment to sustainable healthcare manufacturing.
Working at Hikal provides professionals with exposure to high-value regulatory systems, analytical excellence, and long-term career growth in the pharmaceutical API sector.
Job Role & Responsibilities
Hikal Limited is hiring for multiple positions across Quality Control and API Production departments at its Unit 11 facility in Bangalore.
Quality Control Officer – API
This role supports analytical testing and quality assurance of API products and raw materials in a regulated environment.
Key Responsibilities
- Performing analysis of raw materials, intermediates, and finished API products
- Operating analytical instruments such as HPLC, GC, IR, and auto titrators
- Conducting wet lab analysis and stability studies
- Reviewing analytical data and ensuring compliance with data integrity requirements
- Supporting method validation and routine laboratory activities
- Maintaining GLP-compliant documentation and laboratory records
- Supporting internal audits and regulatory inspections
Experience: 1–4 Years in API Quality Control
Qualification: M.Sc in Chemistry
Key Skills: HPLC, GC, wet lab analysis, raw material testing, IR, auto titrator, stability studies, finished product analysis, data review
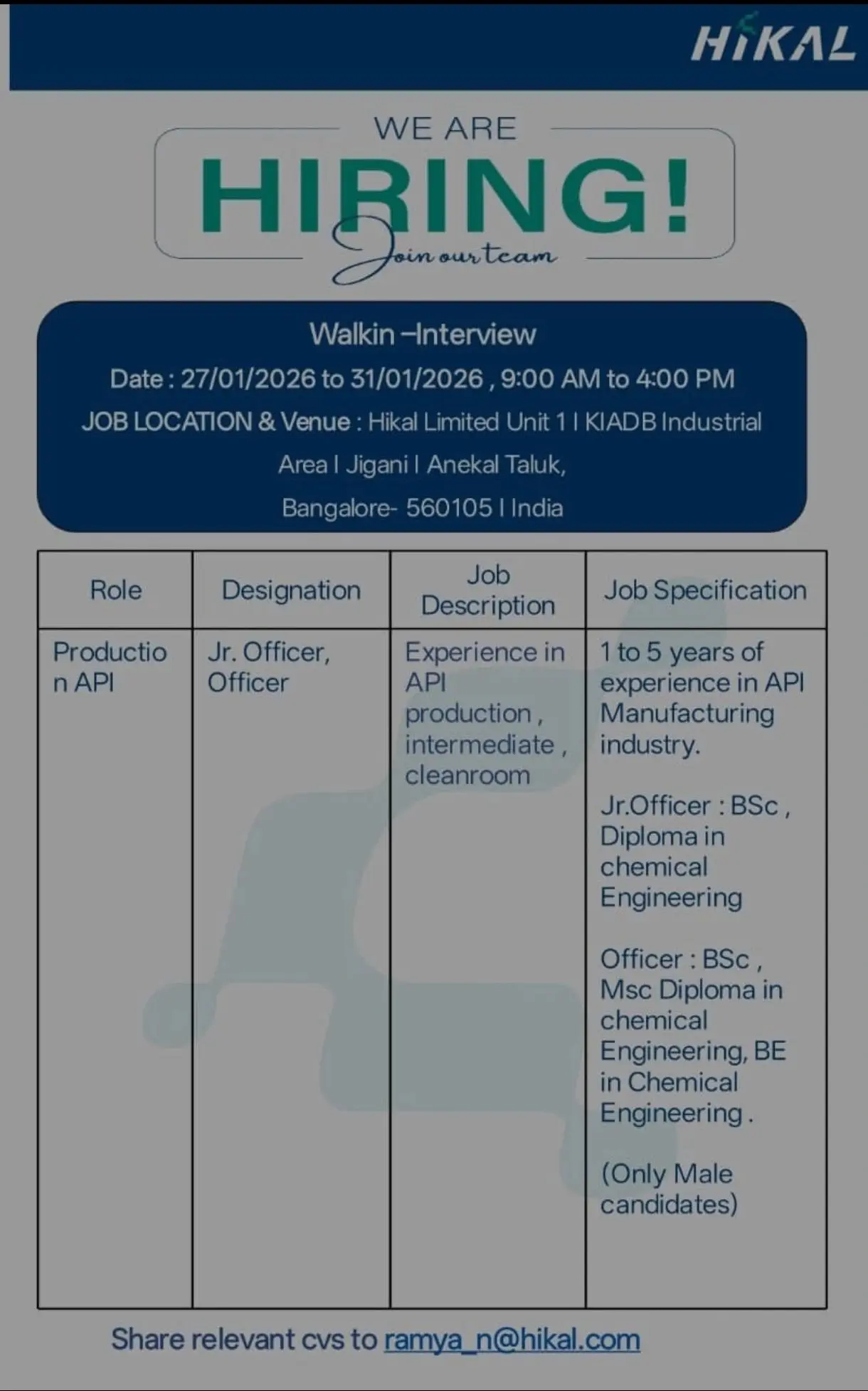
API Production – Junior Officer / Officer
This role focuses on hands-on API manufacturing operations within cleanroom and intermediate production environments.
Key Responsibilities
- Executing API manufacturing processes and intermediate stages
- Operating reactors and associated production equipment
- Working in cleanroom environments following GMP guidelines
- Maintaining batch manufacturing records and production documentation
- Ensuring adherence to safety, quality, and environmental standards
- Coordinating with quality and engineering teams during routine operations
Experience: 1–5 Years in API Manufacturing
Designation & Qualification
- Junior Officer: B.Sc, Diploma in Chemical Engineering
- Officer: B.Sc, M.Sc, Diploma in Chemical Engineering, B.E in Chemical Engineering
Note: Only male candidates are eligible for API Production roles as per current operational requirements.
Eligibility / Qualifications
Required Education
M.Sc Chemistry, B.Sc Chemistry, Diploma in Chemical Engineering, B.E Chemical Engineering
Experience Requirements
- Quality Control Officer: 1–4 years in API QC laboratories
- API Production Jr. Officer / Officer: 1–5 years in API manufacturing environments
Desired Skills
- Strong understanding of GMP and data integrity principles
- Experience in regulated API manufacturing facilities
- Ability to work in shift-based operations
- Good documentation and compliance mindset
Location & Salary
Job Location & Walk-in Venue:
Hikal Limited – Unit 11, KIADB Industrial Area, Jigani, Anekal Taluk, Bangalore – 560105, Karnataka, India
Walk-in Dates & Time:
27 January 2026 to 31 January 2026 | 9:00 AM to 4:00 PM
Salary:
Compensation will be competitive and aligned with industry standards for API quality control and production roles. Final salary will depend on experience, qualification, and interview performance.
Why Build a Career at Hikal Limited
Jobs in API manufacturing and quality control are among the highest CPC categories in pharmaceutical recruitment due to their direct link with regulatory compliance, global supply, and drug safety. Search terms such as API QC jobs, HPLC analyst jobs, API production jobs, and pharmaceutical manufacturing careers consistently attract high-intent candidates.
At Hikal, professionals gain exposure to regulated markets, advanced analytical technologies, and robust quality systems that strengthen long-term career prospects in the pharmaceutical industry.
Application Process
Interested candidates can attend the walk-in interview directly during the scheduled dates.
Candidates may also share their updated CVs to:
- Email: ramya_n@hikal.com
Applicants should carry relevant experience details and be prepared for technical discussions related to API manufacturing or analytical testing.
SEO-Focused Job Highlights
- Hikal Limited Walk-in Interview Bangalore
- API Quality Control Jobs for MSc Chemistry
- API Production Jobs in Pharmaceutical Industry
- HPLC GC Analyst Jobs Pharma
- High Paying API Manufacturing Jobs India
These keywords are integrated naturally to support SEO performance and AdSense monetization without keyword stuffing.
Frequently Asked Questions (FAQs)
Is this walk-in interview open for freshers?
Freshers are not eligible. All roles require prior experience in API quality control or manufacturing.
What qualifications are required for QC roles?
An M.Sc in Chemistry with 1–4 years of API QC experience is mandatory.
Are female candidates eligible for API Production roles?
Currently, API Production roles are open only to male candidates as per operational requirements.
Where is the walk-in interview location?
The walk-in will be conducted at Hikal Limited Unit 11, Jigani, Bangalore.
Can I apply by email?
Yes. Candidates can email their CV to ramya_n@hikal.com.
Career Growth in API Manufacturing and Quality Control
Professionals with API manufacturing and analytical QC experience are in strong demand across regulated pharmaceutical markets. Roles at Hikal can lead to senior analyst, production supervisor, quality assurance, and regulatory-facing positions over time.
By joining Hikal Limited, candidates gain hands-on exposure to regulated API operations that support global healthcare systems and long-term professional growth.
Summary Table
| Category | Details |
|---|---|
| Company | Hikal Limited |
| Vacancies | Quality Control Officer – API, API Production Junior Officer, API Production Officer |
| Required Education | M.Sc Chemistry, B.Sc Chemistry, Diploma in Chemical Engineering, B.E Chemical Engineering |
| Experience | 1–5 Years |

To apply for this job email your details to ramya_n@hikal.com


