NAARI Walk-in Production, QA, QC, R&D & EHS

- Company Overview
- Job Role & Responsibilities
- Production Department
- Engineering Projects Department
- EHS Department
- Analytical R&D Department
- Formulation R&D Department
- Quality Assurance Department
- Quality Control Department
- Eligibility / Qualifications
- Required Education
- Preferred Experience
- Location & Salary
- Why Build a Career at NAARI
- Application Process
- Frequently Asked Questions (FAQs)
- Is regulated plant exposure mandatory?
- Are freshers eligible for this walk-in?
- Is experience in hormones or potent molecules mandatory?
- Where is the job location?
- Career Growth at NAARI
- Summary Table
ITI, B.Pharm Pharma Jobs at NAARI Rudrapur
NAARI hiring Production, QA, QC, R&D & EHS roles in Rudrapur. ITI, B.Pharm, M.Pharm eligible. 2–15 years experience.
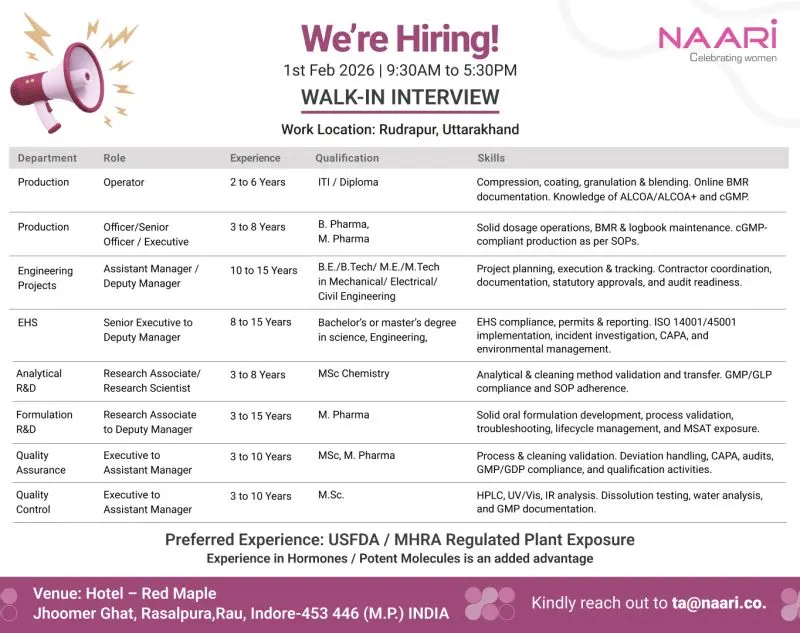
NAARI is conducting a large walk-in interview drive to hire experienced pharmaceutical professionals across Production, Engineering Projects, EHS, Analytical R&D, Formulation R&D, Quality Assurance, and Quality Control. This hiring initiative supports NAARI’s regulated solid dosage manufacturing operations and advanced R&D programs. Candidates with experience in cGMP environments and exposure to regulated plants are invited to attend and build long-term careers with a company committed to quality, compliance, and women-led excellence in healthcare manufacturing.
These roles are designed for professionals who want meaningful work, regulatory exposure, and growth opportunities within a compliance-driven pharmaceutical organization.
Company Overview
NAARI is a pharmaceutical organization focused on delivering high-quality medicines while promoting inclusive growth and women-centric leadership. The company operates regulated manufacturing facilities with strong adherence to global quality standards such as cGMP, data integrity, and audit readiness.
NAARI’s operations span solid oral dosage manufacturing, advanced analytical and formulation R&D, and robust quality systems. With increasing demand from regulated markets, the company continues to invest in skilled professionals who can support compliant production, innovation, and patient safety.
Working at NAARI offers professionals exposure to regulated manufacturing practices, lifecycle product management, and a culture that values responsibility, collaboration, and long-term career development.
Job Role & Responsibilities
NAARI is hiring for multiple departments through this walk-in interview. Each role contributes directly to safe, compliant pharmaceutical manufacturing and product development.
Production Department
Operator – Production
Experience: 2 to 6 Years
Qualification: ITI, Diploma
Key Responsibilities
- Operating solid dosage equipment including granulation, blending, compression, and coating
- Performing online BMR documentation and logbook maintenance
- Ensuring adherence to ALCOA and ALCOA+ data integrity principles
- Following cGMP guidelines and SOPs during manufacturing activities
- Supporting line clearance, changeover, and routine production operations
Officer / Senior Officer / Executive – Production
Experience: 3 to 8 Years
Qualification: B.Pharm, M.Pharm
Key Responsibilities
- Supervising solid dosage manufacturing operations
- Reviewing batch manufacturing records and production documentation
- Ensuring cGMP-compliant production as per approved SOPs
- Coordinating with QA, QC, and engineering teams
- Supporting regulatory audits and inspection readiness
Engineering Projects Department
Assistant Manager / Deputy Manager – Projects
Experience: 10 to 15 Years
Qualification: B.E, B.Tech, M.E, M.Tech in Mechanical, Electrical, or Civil Engineering
Key Responsibilities
- Project planning, execution, and progress tracking
- Contractor coordination and site supervision
- Managing technical documentation and statutory approvals
- Ensuring audit readiness and compliance with engineering standards
EHS Department
Senior Executive to Deputy Manager – EHS
Experience: 8 to 15 Years
Qualification: Bachelor’s or Master’s degree in Science or Engineering
Key Responsibilities
- Managing EHS compliance, permits, and statutory reporting
- Implementing ISO 14001 and ISO 45001 systems
- Incident investigation, CAPA implementation, and risk assessments
- Environmental management and workplace safety initiatives
Analytical R&D Department
Research Associate / Research Scientist – Analytical R&D
Experience: 3 to 8 Years
Qualification: M.Sc Chemistry
Key Responsibilities
- Analytical method development, validation, and transfer
- Cleaning method validation and routine analysis
- Ensuring GMP and GLP compliance in analytical activities
- Preparing and reviewing SOPs and technical documentation
Formulation R&D Department
Research Associate to Deputy Manager – Formulation R&D
Experience: 3 to 15 Years
Qualification: M.Pharm
Key Responsibilities
- Solid oral formulation development and optimization
- Process validation and troubleshooting
- Product lifecycle management and scale-up support
- MSAT exposure and technology transfer activities
Quality Assurance Department
Executive to Assistant Manager – Quality Assurance
Experience: 3 to 10 Years
Qualification: M.Sc, M.Pharm
Key Responsibilities
- Process and cleaning validation activities
- Handling deviations, CAPA, and change controls
- Supporting internal and external audits
- Ensuring GMP and GDP compliance
Quality Control Department
Executive to Assistant Manager – Quality Control
Experience: 3 to 10 Years
Qualification: M.Sc
Key Responsibilities
- Performing analysis using HPLC, UV/Vis, and IR instruments
- Dissolution testing and water analysis
- Maintaining GMP-compliant documentation
- Supporting laboratory investigations and audit readiness
Eligibility / Qualifications
Required Education
ITI, Diploma, B.Pharm, M.Pharm, M.Sc Chemistry, B.E, B.Tech, M.E, M.Tech, Bachelor’s or Master’s degree in Science or Engineering
Preferred Experience
- Exposure to USFDA or MHRA regulated manufacturing plants
- Experience in Hormones or Potent Molecule manufacturing is an added advantage
- Strong understanding of cGMP, data integrity, and audit compliance
Location & Salary
Work Location: Rudrapur, Uttarakhand
Walk-in Venue:
Hotel Red Maple, Jhoomer Ghat, Rasalpura, Rau, Indore – 453446, Madhya Pradesh
Date & Time:
01 February 2026 | 9:30 AM to 5:30 PM
Salary:
Compensation will be competitive and aligned with industry standards based on role, experience, and regulatory exposure. Salary details will be discussed during the interview.
Why Build a Career at NAARI
Pharma roles in regulated solid dosage plants attract high CPC search demand due to compliance complexity and regulatory exposure. Keywords such as pharma production jobs, QA QC jobs in pharmaceutical companies, formulation R&D jobs, analytical R&D careers, and EHS jobs in pharma are among the highest-paying search segments.
At NAARI, professionals contribute directly to healthcare advancement while building expertise in regulated manufacturing, quality systems, and product innovation.
Application Process
Interested candidates can attend the walk-in interview at the venue on the specified date and time.
Candidates may also reach out to:
- Email: ta@naari.co
Applicants are advised to carry updated resumes and relevant experience details.
Frequently Asked Questions (FAQs)
Is regulated plant exposure mandatory?
Regulated plant exposure such as USFDA or MHRA is preferred and strongly valued but not mandatory for all roles.
Are freshers eligible for this walk-in?
Freshers may be considered only for select production operator roles. Most positions require prior experience.
Is experience in hormones or potent molecules mandatory?
No. It is an added advantage but not mandatory.
Where is the job location?
The job location is Rudrapur, Uttarakhand.
Career Growth at NAARI
Careers at NAARI offer exposure to regulated pharmaceutical manufacturing, advanced R&D, and robust quality systems. Employees gain long-term growth opportunities in production leadership, quality management, R&D specialization, and compliance roles.
By joining NAARI, professionals become part of a healthcare-driven organization that values quality, integrity, and inclusive growth.
Summary Table
| Category | Details |
|---|---|
| Company | NAARI |
| Vacancies | Production Operator, Production Officer/Sr. Officer/Executive, Engineering Projects Asst. Manager/Deputy Manager, EHS Senior Executive/Deputy Manager, Analytical R&D Research Associate/Scientist, Formulation R&D Research Associate/Deputy Manager, QA Executive/Asst. Manager, QC Executive/Asst. Manager |
| Required Education | ITI, Diploma, B.Pharm, M.Pharm, M.Sc Chemistry, B.E/B.Tech, M.E/M.Tech |
| Experience | 2–15 Years (Role dependent) |
To apply for this job email your details to ta@naari.co


