Lupin walk-in QA QC

- Company Overview
- Job Role & Responsibilities
- Quality Control (QC)
- Quality Assurance – QMS
- Quality Assurance – IPQA
- Quality Assurance – Validation
- Eligibility / Qualifications
- Educational Qualifications
- Experience Requirements
- Mandatory Criteria
- Location & Salary
- Job Location
- Interview Venue
- Walk-In Interview Details
- Date
- Time
- Application Process
- Why Build Your QA/QC Career with Lupin
- Frequently Asked Questions (FAQs)
- Who can attend this Lupin walk-in drive?
- Is OSD experience mandatory?
- Are freshers eligible?
- Can female candidates apply?
- SEO Title Variations for Higher Visibility
- Summary Table
BPharm MSc QA QC Vacancies – Lupin Sambhajinagar
BPharm, MPharm, MSc QA QC vacancies at Lupin Sambhajinagar. Officer & Executive roles, 2–12 years experience.
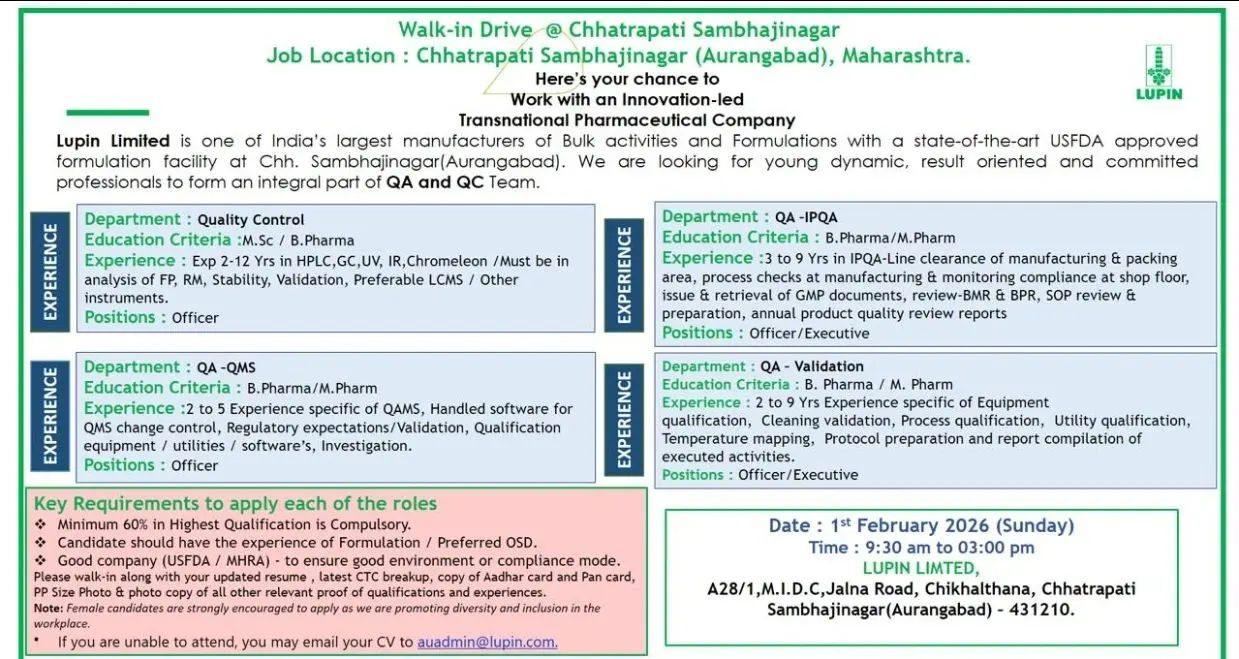
Lupin Limited has announced a walk-in drive at its USFDA-approved formulation facility in Chhatrapati Sambhajinagar (Aurangabad), Maharashtra. This hiring initiative targets experienced Quality Assurance and Quality Control professionals who want to work with a transnational, innovation-driven pharmaceutical company known for strong compliance culture and global regulatory exposure. The opportunity is ideal for candidates with formulation and OSD experience looking to advance their careers in regulated pharma manufacturing.
Company Overview
Lupin Limited is one of India’s largest pharmaceutical companies with a strong global presence across regulated and emerging markets. The company manufactures bulk drugs and finished formulations and operates multiple USFDA, MHRA, and WHO-GMP compliant facilities worldwide.
Lupin’s Chhatrapati Sambhajinagar formulation plant is a state-of-the-art manufacturing unit known for its robust quality systems, audit readiness, and focus on continuous improvement. Working at Lupin offers exposure to international regulatory expectations, advanced analytical systems, and long-term career growth in pharmaceutical quality functions.
Job Role & Responsibilities
Lupin is hiring across multiple QA and QC functions. Candidates must have relevant experience in formulation, preferably oral solid dosage (OSD).
Quality Control (QC)
Position: Officer
Experience: 2 to 12 Years
Key Responsibilities:
- Performing analysis of finished products, raw materials, and stability samples
- Operating and troubleshooting analytical instruments such as HPLC, GC, UV, IR, and Chromeleon
- Supporting method validation and analytical validation activities
- Handling stability studies and data interpretation
- Maintaining GLP documentation and audit trails
- Preferable exposure to LCMS and other advanced analytical instruments
Quality Assurance – QMS
Position: Officer
Experience: 2 to 5 Years
Key Responsibilities:
- Managing Quality Management System (QMS) activities
- Handling change control, deviations, and CAPA
- Supporting regulatory inspections and audits
- Managing validation and qualification documentation
- Handling QMS software and compliance tracking systems
Quality Assurance – IPQA
Positions: Officer / Executive
Experience: 3 to 9 Years
Key Responsibilities:
- Performing line clearance in manufacturing and packing areas
- Monitoring shop floor compliance and process checks
- Issuance and retrieval of GMP documents
- Reviewing BMRs, BPRs, SOPs, and APQR reports
- Supporting investigations and continuous improvement initiatives
Quality Assurance – Validation
Positions: Officer / Executive
Experience: 2 to 9 Years
Key Responsibilities:
- Executing equipment qualification and process validation
- Performing cleaning validation and utility qualification
- Conducting temperature mapping studies
- Preparing and reviewing validation protocols and reports
- Supporting regulatory audits related to validation activities
Eligibility / Qualifications
Educational Qualifications
Quality Control:
MSc, BPharm
Quality Assurance:
BPharm, MPharm
Relevant courses include:
BPharm, MPharm (Quality Assurance), MSc Chemistry, MSc Pharmaceutical Chemistry
Experience Requirements
- Minimum 2 years of relevant QA/QC experience
- Maximum experience up to 12 years depending on role
- Experience in formulation manufacturing; OSD preferred
- Candidates must have worked in regulated companies (USFDA/MHRA environments)
Mandatory Criteria
- Minimum 60% marks in highest qualification
- Strong documentation and compliance mindset
Female candidates are strongly encouraged to apply as part of Lupin’s diversity and inclusion initiative.
Location & Salary
Job Location
Chhatrapati Sambhajinagar (Aurangabad), Maharashtra
Interview Venue
Lupin Limited
A-28/1, MIDC, Jalna Road
Chikhalthana
Chhatrapati Sambhajinagar – 431210
Salary details are not disclosed and will be offered as per industry standards, role, and experience.
Walk-In Interview Details
Date
01 February 2026 (Sunday)
Time
09:30 AM to 03:00 PM
Application Process
Eligible candidates should attend the walk-in interview with the following documents:
- Updated resume
- Latest CTC breakup
- Copy of Aadhaar and PAN card
- Passport-size photograph
- Photocopies of educational and experience certificates
Candidates unable to attend the walk-in may send their CV to:
Email ID: auadmin@lupin.com
Why Build Your QA/QC Career with Lupin
- Work with a globally respected pharmaceutical organization
- Exposure to USFDA and MHRA regulated manufacturing
- Strong quality culture and compliance-driven environment
- Opportunities for career growth in QA and QC leadership roles
- Direct contribution to safe and effective medicines
Frequently Asked Questions (FAQs)
Who can attend this Lupin walk-in drive?
Candidates with QA or QC experience in formulation manufacturing can attend.
Is OSD experience mandatory?
OSD experience is preferred for most roles.
Are freshers eligible?
No. These roles are strictly for experienced professionals.
Can female candidates apply?
Yes. Female candidates are strongly encouraged to apply.
SEO Title Variations for Higher Visibility
- Lupin QA QC Walk-In Sambhajinagar (Aurangabad)
- BPharm MSc QA QC Jobs at Lupin Maharashtra
- Pharmaceutical Quality Jobs at Lupin Formulation Plant
Summary Table
| Category | Details |
|---|---|
| Company | Lupin Limited |
| Vacancies | QC Officer, QA-QMS Officer, QA-IPQA Officer/Executive, QA-Validation Officer/Executive |
| Required Education | BPharm, MPharm, MSc |
| Experience | 2 to 12 Years |