Ceph Lifesciences Hiring QA QC Production, Engineering

- Company Overview
- Job Role & Responsibilities
- Production Department
- Quality Assurance Department
- Quality Control Department
- Engineering Department
- Eligibility / Qualifications
- Educational Qualifications
- Experience Requirement
- Location & Salary
- Job Location
- Application Process
- Contact Numbers
- Why Build Your Pharma Career with Ceph Lifesciences
- Frequently Asked Questions (FAQs)
- Who can apply for Ceph Lifesciences vacancies?
- Are freshers eligible?
- Where is the job location?
- How can I apply?
- SEO Title Variations for Higher Visibility
- Summary Table
BPharm QA QC Production Vacancies – Ceph Baddi
BPharm, MPharm QA QC Production vacancies at Ceph Lifesciences Baddi. Multiple roles, 1–13 years experience.
Ceph Lifesciences has announced multiple job openings across Production, Quality Assurance, Quality Control, and Engineering departments at its manufacturing facility located in Baddi, Himachal Pradesh. This recruitment drive is aimed at experienced pharmaceutical professionals seeking long-term careers in regulated formulation and injectable manufacturing environments. With opportunities ranging from operators to managerial roles, Ceph offers a stable and compliance-driven workplace for skilled candidates.
Company Overview
Ceph Lifesciences is a pharmaceutical manufacturing organization engaged in the production of oral solid dosage forms and sterile injectable products. The company operates GMP-compliant facilities and follows strict quality and regulatory standards to ensure the safety, efficacy, and consistency of its products.
Located in Baddi, one of India’s major pharmaceutical hubs, Ceph Lifesciences supports domestic and export markets through disciplined manufacturing practices, strong documentation systems, and a quality-first culture. Working at Ceph provides exposure to regulated plant operations, audits, and long-term professional growth in pharmaceutical manufacturing and quality functions.
Job Role & Responsibilities
Ceph Lifesciences is hiring across multiple departments as outlined below.
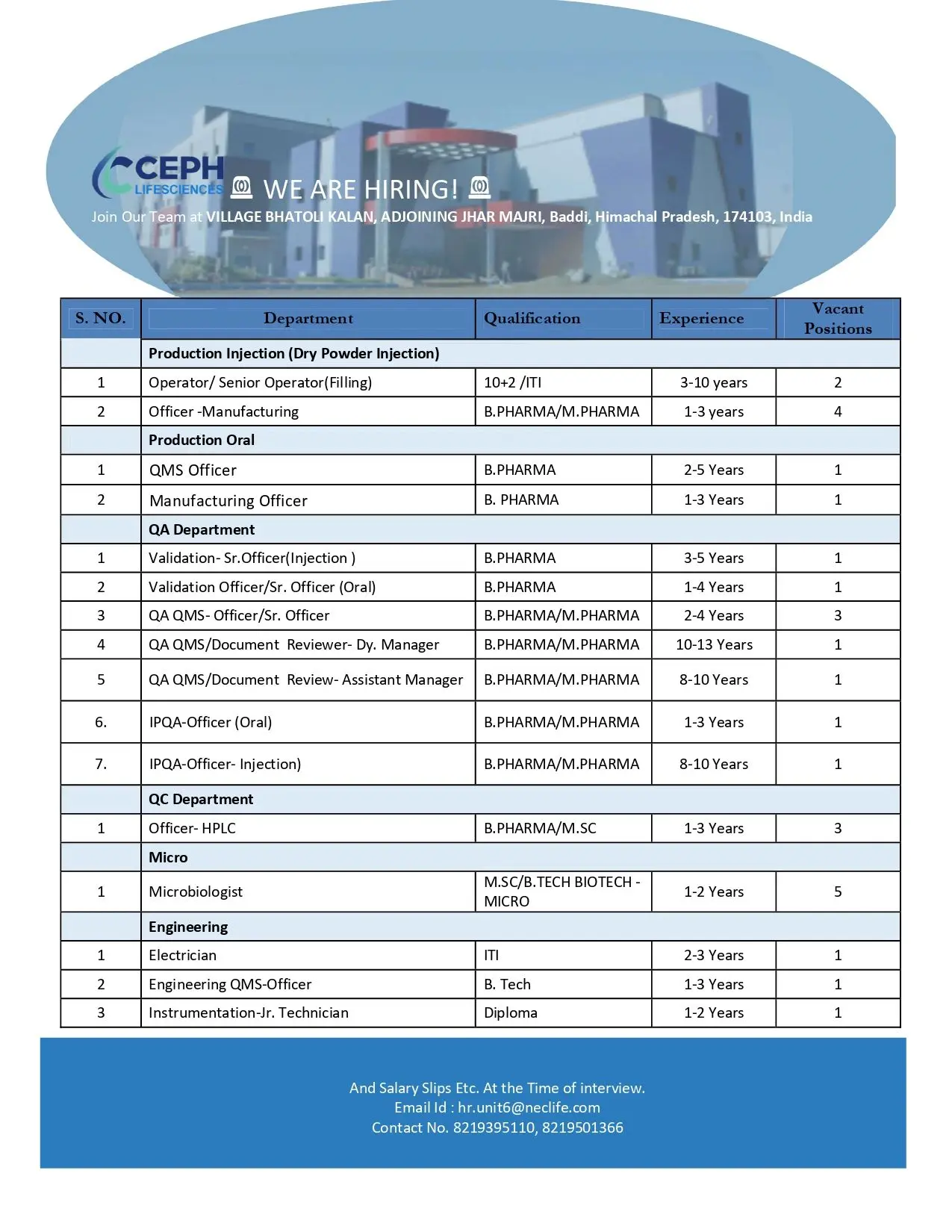
Production Department
Injectables – Dry Powder Injection
Positions: Operator, Senior Operator (Filling), Officer – Manufacturing
Qualifications: BPharm, MPharm
Experience: 1 to 3 Years
Key Responsibilities:
- Operating and monitoring filling operations for dry powder injectables
- Following aseptic techniques and GMP guidelines
- Maintaining batch manufacturing records and documentation
- Supporting compounding, filling, and line clearance activities
Oral Manufacturing
Positions: Manufacturing Officer, QMS Officer
Qualifications: 10+2, ITI, BPharm
Experience: 2 to 10 Years
Key Responsibilities:
- Executing oral solid dosage manufacturing activities
- Supporting QMS documentation and compliance
- Coordinating with QA and QC teams
- Ensuring adherence to SOPs and cGMP requirements
Quality Assurance Department
Validation (Injectables)
Position: Senior Officer
Qualification: BPharm
Experience: 3 to 5 Years
Responsibilities:
- Equipment and process validation for injectable lines
- Cleaning validation and documentation
- Supporting regulatory inspections
Validation (Oral)
Position: Officer / Senior Officer
Qualification: BPharm
Experience: 1 to 4 Years
QA QMS / Documentation Review
Positions: Officer, Senior Officer, Assistant Manager, Deputy Manager
Qualification: BPharm, MPharm
Experience: 2 to 13 Years
Responsibilities:
- Handling QMS, document control, and review activities
- Managing deviations, CAPA, and change control
- Supporting audits and compliance activities
IPQA (Oral & Injection)
Positions: Officer
Qualification: BPharm, MPharm
Experience: 1 to 10 Years
Responsibilities:
- In-process quality assurance activities
- Line clearance and shop floor compliance
- Review of BMR and BPR documents
Quality Control Department
Positions: Officer – HPLC, Microbiologist
Qualifications: BPharm, MSc, BTech Biotechnology
Experience: 1 to 3 Years
Responsibilities:
- Performing analytical and microbiological testing
- Handling HPLC instruments and microbiology labs
- Maintaining GLP and cGMP documentation
Engineering Department
Positions: Electrician, Instrumentation Technician, Engineering QMS Officer
Qualifications: Diploma, BTech
Experience: 1 to 3 Years
Responsibilities:
- Maintenance of plant equipment and utilities
- Supporting instrumentation and calibration
- Engineering documentation and compliance
Eligibility / Qualifications
Educational Qualifications
10+2, ITI, Diploma, BPharm, MPharm, MSc, BTech
Relevant courses include:
BPharm, MPharm, MSc Chemistry, MSc Microbiology, BTech Biotechnology, Diploma in Engineering, ITI Trades
Experience Requirement
- Experience ranges from 1 to 13 years depending on role
- Prior pharmaceutical manufacturing experience is mandatory
Location & Salary
Job Location
Ceph Lifesciences
Village Bratoli Kalan
Adjoining Jhar Majri
Baddi, Himachal Pradesh – 174103
Salary details are not disclosed and will be offered as per company standards and candidate experience.
Application Process
Interested candidates should attend the interview with updated resume, salary slips, and relevant documents.
Candidates can also send their CV via email:
Email ID: hr.unita@neclife.com
Contact Numbers
8219395110, 8219501366
Why Build Your Pharma Career with Ceph Lifesciences
- Work in a GMP-compliant formulation and injectables facility
- Exposure to oral and sterile manufacturing operations
- Strong quality and documentation culture
- Opportunities across technical and managerial roles
- Stable employment in a major pharma hub
Frequently Asked Questions (FAQs)
Who can apply for Ceph Lifesciences vacancies?
Candidates with relevant pharma experience and required qualifications can apply.
Are freshers eligible?
No. These roles require prior pharmaceutical experience.
Where is the job location?
The job location is Baddi, Himachal Pradesh.
How can I apply?
Attend the interview or send your CV to hr.unita@neclife.com.
SEO Title Variations for Higher Visibility
- Ceph Lifesciences Hiring QA QC Production Baddi
- BPharm MPharm Pharma Jobs in Baddi Himachal Pradesh
- Injectables and Oral Manufacturing Vacancies at Ceph
Summary Table
| Category | Details |
|---|---|
| Company | Ceph Lifesciences |
| Vacancies | Production, QA, QC, Engineering (Multiple Positions) |
| Required Education | 10+2, ITI, Diploma, BPharm, MPharm, MSc, BTech |
| Experience | 1 to 13 Years |
To apply for this job email your details to hr.unita@neclife.com


