Corona Walk-in Production, QA, QC

- Company Overview
- Job Role & Responsibilities
- Production Department
- Quality Assurance Department
- Quality Control Department
- Eligibility / Qualifications
- Required Education
- Experience Criteria
- Location & Salary
- Walk-in Interview Details
- Application Process (If Unable to Attend Walk-in)
- Why Build Your Career with Corona Remedies Limited
- SEO-Optimized Job Titles
- Frequently Asked Questions (FAQs)
- Who can apply for Corona Remedies walk-in interview?
- Are freshers eligible for these vacancies?
- What manufacturing exposure is required?
- Is this a walk-in or online interview?
- What if I cannot attend the walk-in interview?
- Summary Table
BPharm MSc Pharma Vacancies at Corona Remedies Ahmedabad
Corona Remedies hiring BPharm, MSc candidates for Production, QA, QC roles in Ahmedabad. Walk-in vacancies for experienced professionals.
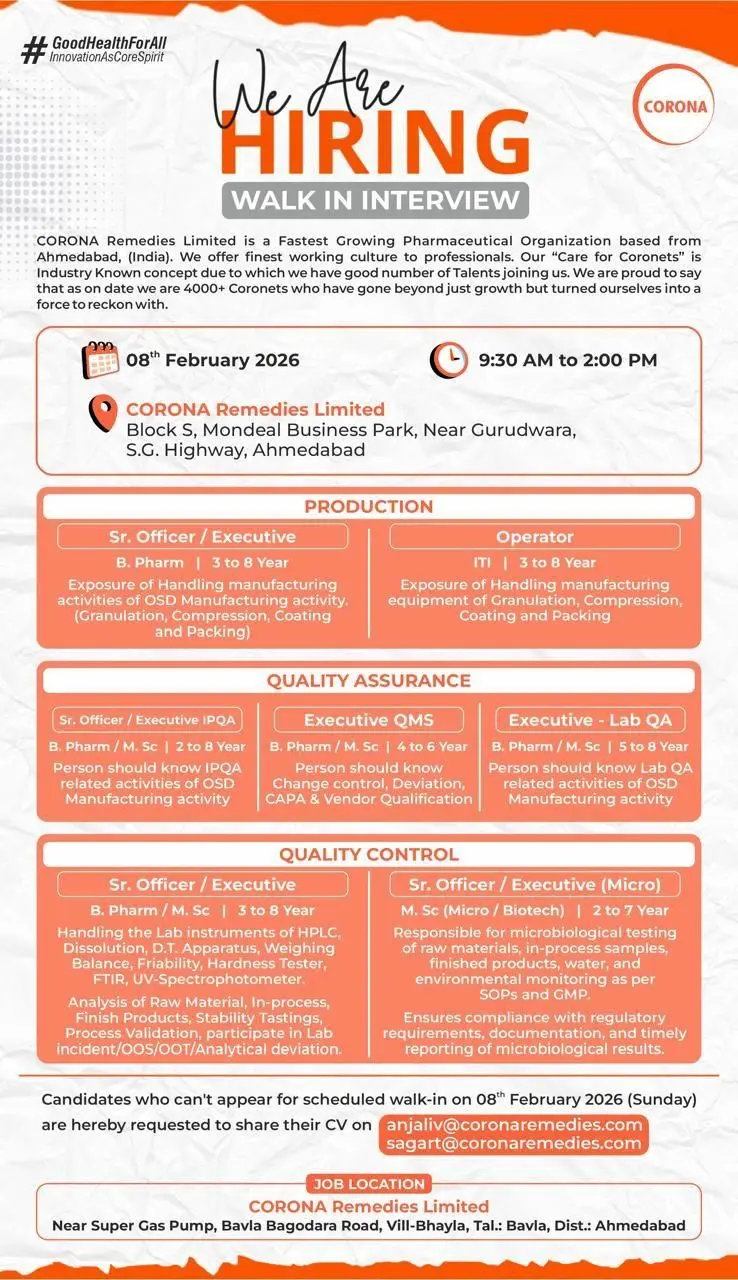
Corona Remedies Limited has announced a large-scale walk-in interview for experienced pharmaceutical professionals across Production, Quality Assurance, and Quality Control departments. This hiring drive is scheduled for 08 February 2026 at Ahmedabad and is aimed at strengthening the company’s oral solid dosage (OSD) manufacturing and quality operations. The openings are suitable for B.Pharm, M.Sc, and Microbiology professionals with hands-on exposure to regulated pharma manufacturing environments.
Company Overview
Corona Remedies Limited is one of India’s fastest-growing pharmaceutical companies, headquartered in Ahmedabad. The organization is widely recognized for its strong presence in branded formulations and its people-centric work culture. With a workforce of over 4,000 professionals, known internally as “Coronets,” the company has built a reputation for sustainable growth, ethical practices, and continuous investment in talent development.
Corona Remedies operates modern manufacturing facilities that comply with national and international regulatory standards. The company’s focus on quality systems, patient-centric healthcare solutions, and operational excellence has positioned it as a trusted name in the Indian pharmaceutical industry. Joining Corona Remedies provides professionals with exposure to advanced OSD manufacturing technologies, robust quality systems, and long-term career stability.
Job Role & Responsibilities
Production Department
Sr. Officer / Executive – Production
Experience: 3 to 8 Years
Key Responsibilities:
- Handling end-to-end OSD manufacturing activities
- Operation and supervision of granulation, compression, coating, and packing processes
- Ensuring adherence to batch manufacturing records and GMP guidelines
- Coordination with QA and engineering during production operations
- Troubleshooting manufacturing issues and maintaining productivity targets
Officer / Executive – Production (IPQA / POA)
Experience: 2 to 7 Years
Key Responsibilities:
- Handling in-process quality activities related to OSD manufacturing
- Monitoring critical process parameters during granulation, compression, and coating
- Ensuring GMP compliance and documentation accuracy
- Coordination with QA for deviations and corrective actions
Operator – Production
Experience: 0 to 3 Years
Key Responsibilities:
- Supporting manufacturing activities in granulation, compression, coating, and packing
- Equipment operation under supervision
- Maintaining cleanliness and GMP standards on shop floor
- Documentation support and material handling
Quality Assurance Department
Executive – QMS
Experience: 4 to 6 Years
Key Responsibilities:
- Handling Quality Management System activities
- Change control, deviation management, and CAPA implementation
- Vendor qualification and audit support
- Preparation, review, and maintenance of QMS documentation
Executive – Lab QA
Experience: 5 to 8 Years
Key Responsibilities:
- Oversight of laboratory quality assurance activities
- Review of analytical data and lab documentation
- Ensuring compliance with GMP and regulatory guidelines
- Support during audits, inspections, and investigations
Quality Control Department
Sr. Officer / Executive – Quality Control (Chemical)
Experience: 3 to 8 Years
Key Responsibilities:
- Operation and maintenance of analytical instruments such as HPLC, Dissolution, UV, FTIR
- Analysis of raw materials, in-process samples, finished products, and stability samples
- Participation in process validation activities
- Handling OOS, OOT, analytical deviations, and lab incidents
- Documentation and data integrity compliance
Sr. Officer / Executive – Quality Control (Microbiology)
Experience: 2 to 7 Years
Key Responsibilities:
- Microbiological testing of raw materials, in-process samples, finished products, and water
- Environmental monitoring and microbial limit testing
- Compliance with GMP and regulatory microbiology standards
- Documentation, reporting, and investigation support
Eligibility / Qualifications
Required Education
- B.Pharm
- M.Sc (Chemistry, Pharmaceutical Chemistry)
- M.Sc Microbiology
- M.Sc Biotechnology
Relevant Courses Include:
B.Pharm, M.Pharm, M.Sc Chemistry, M.Sc Pharmaceutical Chemistry, M.Sc Microbiology, M.Sc Biotechnology
Experience Criteria
- Experience ranges from 0 to 8 years depending on the role
- Prior exposure to OSD manufacturing and regulated environments is mandatory for senior roles
Location & Salary
Job Location:
Corona Remedies Limited
Near Super Gas Pump, Bavla Bagodara Road,
Village Bhayla, Taluka Bavla, District Ahmedabad
Salary Package:
Salary will be competitive and aligned with industry standards, based on qualification, experience, and interview performance.
Walk-in Interview Details
Date: 08 February 2026 (Sunday)
Time: 9:30 AM to 2:00 PM
Venue:
Block S, Mondeal Business Park,
Near Gurudwara, S.G. Highway, Ahmedabad
Candidates are requested to carry updated CV, educational certificates, experience letters, and recent passport-size photographs.
Application Process (If Unable to Attend Walk-in)
Candidates who are unable to attend the scheduled walk-in interview may share their updated CV via email.
Email IDs:
anjaliv@coronaremedies.com
sagart@coronaremedies.com
Why Build Your Career with Corona Remedies Limited
- Fast-growing pharmaceutical organization with strong market presence
- Exposure to regulated OSD manufacturing and advanced quality systems
- Structured career growth and learning opportunities
- Employee-focused culture with long-term stability
- Direct contribution to affordable and quality healthcare solutions
SEO-Optimized Job Titles
- Corona Remedies Production, QA, QC Vacancies in Ahmedabad
- BPharm MSc Jobs in Pharma Manufacturing at Corona Remedies
- Corona Remedies Walk-in Interview for Experienced Pharma Professionals
- OSD Manufacturing Jobs in Ahmedabad – Corona Remedies Limited
Frequently Asked Questions (FAQs)
Who can apply for Corona Remedies walk-in interview?
Candidates with B.Pharm or M.Sc qualifications and relevant pharma manufacturing or quality experience can apply.
Are freshers eligible for these vacancies?
Freshers are eligible only for operator-level roles in the production department.
What manufacturing exposure is required?
Candidates should have hands-on exposure to OSD manufacturing, quality assurance, or quality control activities.
Is this a walk-in or online interview?
This is a direct walk-in interview scheduled on 08 February 2026.
What if I cannot attend the walk-in interview?
Candidates may share their CV via the provided official email IDs for consideration.
Summary Table
| Company | Corona Remedies Limited |
|---|---|
| Vacancies | Sr. Officer/Executive – Production, Officer/Executive – Production (IPQA/POA), Operator – Production, Executive – QMS, Executive – Lab QA, Sr. Officer/Executive – QC (Chemical), Sr. Officer/Executive – QC (Microbiology) |
| Required Education | B.Pharm, M.Pharm, M.Sc Chemistry, M.Sc Pharmaceutical Chemistry, M.Sc Microbiology, M.Sc Biotechnology |
| Experience | 0 to 8 Years |
To apply for this job email your details to sagart@coronaremedies.com


