IPCA Walk-in QA, QC, Production

- Company Overview
- Job Role & Responsibilities
- Quality Assurance Department
- Quality Control Department
- Production Department
- Engineering Department
- Eligibility / Qualifications
- Required Education
- Experience Criteria
- Location & Salary
- Walk-in Interview Details
- Documents to Carry
- Important Notes
- Why Join IPCA Laboratories Limited
- SEO-Optimized Job Titles
- Frequently Asked Questions (FAQs)
- Who can apply for IPCA Laboratories Athal walk-in interview?
- Are freshers eligible for these vacancies?
- What manufacturing experience is required?
- Is shift duty mandatory?
- What is the interview venue?
- Summary Table
ITI BPharm QA QC Production Jobs at IPCA Athal
IPCA Laboratories hiring ITI, Diploma, BPharm, MSc candidates for QA, QC, Production roles at Athal (Silvassa). Walk-in vacancies.
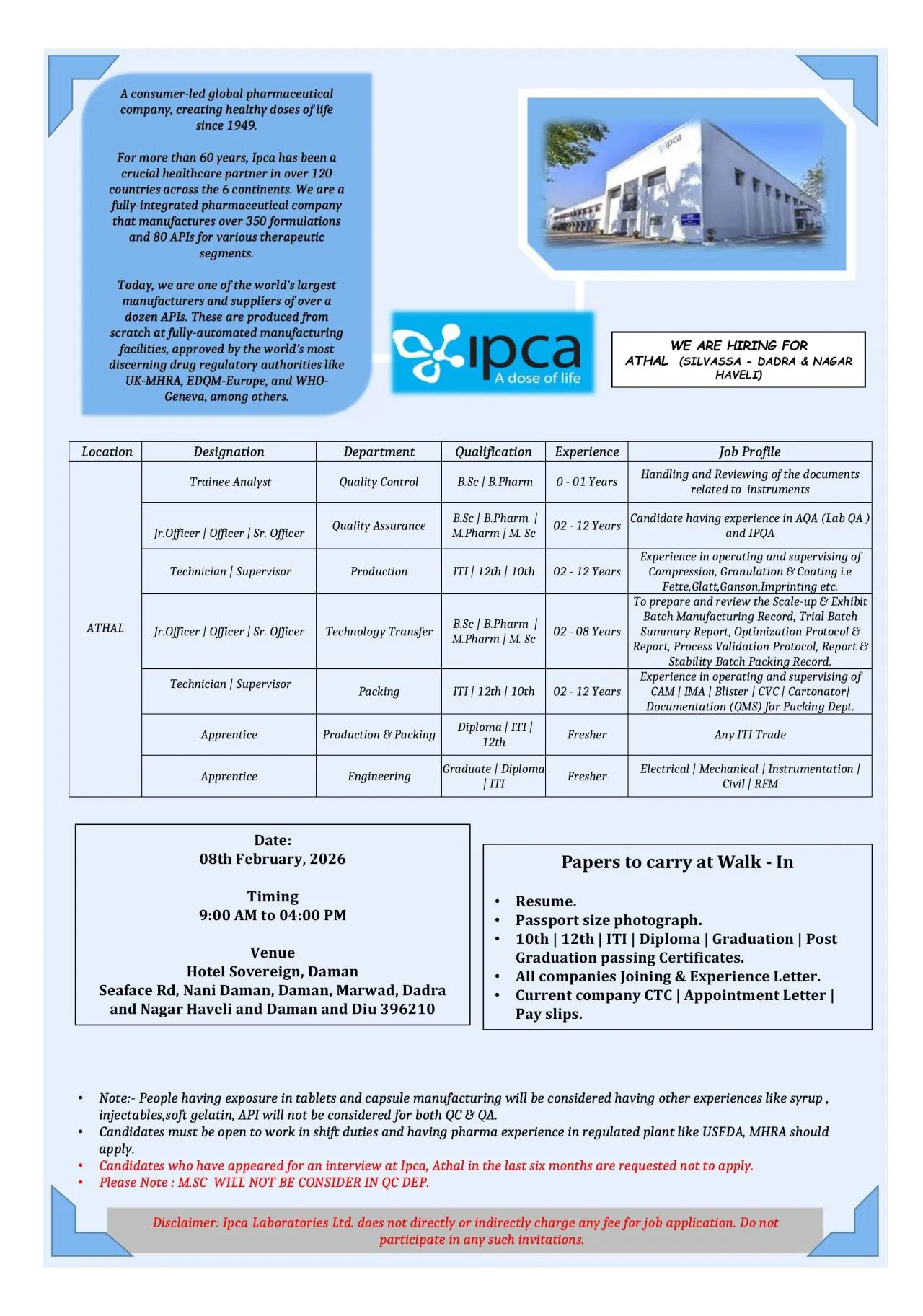
IPCA Laboratories Limited has announced a large walk-in interview drive for its Athal manufacturing facility located in Silvassa (Dadra & Nagar Haveli). This hiring initiative targets experienced and fresher candidates across Quality Assurance, Quality Control, Production, Packing, and Engineering departments. The openings are ideal for candidates seeking long-term careers in a globally regulated pharmaceutical manufacturing environment with strong exposure to USFDA, MHRA, and other international regulatory standards.
Company Overview
IPCA Laboratories Limited is a globally recognized, fully integrated pharmaceutical company with more than six decades of experience in healthcare. The organization operates across over 120 countries and manufactures a wide portfolio of finished dosage formulations and Active Pharmaceutical Ingredients (APIs) spanning multiple therapeutic segments. IPCA is among the world’s leading manufacturers of APIs, supported by fully automated and regulator-approved manufacturing facilities.
The Athal facility is a critical production unit focused on solid oral dosage forms, including tablets and capsules. Approved by global regulatory authorities such as USFDA, MHRA (UK), EU GMP, and WHO, the plant follows stringent quality systems, data integrity practices, and compliance-driven manufacturing operations. Working at IPCA Laboratories provides professionals with exposure to world-class pharma manufacturing, audit readiness, and global healthcare supply chains.
Job Role & Responsibilities
Quality Assurance Department
Trainee / Officer / Senior Officer – Quality Assurance
Experience: 0 to 2 Years
Key Responsibilities:
- Handling and reviewing GMP documentation related to manufacturing and quality systems
- Supporting IPQA activities during tablet and capsule manufacturing
- Review of batch manufacturing records and trial batch documentation
- Handling ALCOA+ compliant data integrity practices
- Support during audits, inspections, and deviation management
- Ensuring compliance with cGMP and regulatory guidelines
Quality Control Department
Trainee / Officer – Quality Control
Experience: 0 to 2 Years
Key Responsibilities:
- Sampling and analysis of raw materials and in-process samples
- Operation of analytical instruments as per SOPs
- Documentation and review of analytical data
- Compliance with GLP and laboratory quality systems
- Support during method transfer and routine analysis
Production Department
Technical Supervisor – Production
Experience: 2 to 8 Years
Key Responsibilities:
- Supervising tablet manufacturing processes including granulation, compression, and coating
- Preparation and review of batch manufacturing records
- Technology transfer and execution of trial batches
- Ensuring productivity, quality, and compliance on shop floor
- Coordination with QA and QC teams
Technician / Operator – Production & Packing
Experience: Fresher to Experienced
Key Responsibilities:
- Operation and supervision of tablet compression, coating, and packing equipment
- Handling blister packing, cartonator, and CAM machines
- Line clearance, logbook maintenance, and documentation
- Compliance with GMP and safety requirements
- Willingness to work in shift duties
Engineering Department
Technician – Engineering
Experience: Fresher
Key Responsibilities:
- Maintenance and troubleshooting of electrical, mechanical, and instrumentation systems
- Supporting plant utilities and equipment upkeep
- Compliance with safety and engineering SOPs
Eligibility / Qualifications
Required Education
- ITI (Any Trade)
- Diploma (Engineering)
- B.Pharm
- M.Pharm
- B.Sc
Relevant Courses Include:
ITI Electrician, ITI Mechanical, ITI Instrumentation, Diploma Mechanical, Diploma Electrical, B.Pharm, M.Pharm, B.Sc Chemistry
Experience Criteria
- Fresher to 8 years of experience depending on the role
- Mandatory exposure to tablet and capsule manufacturing for QA and QC roles
- Candidates with syrup, injectables, soft gelatin, or API-only experience will not be considered
- Willingness to work in shift duties
Location & Salary
Job Location:
IPCA Laboratories Limited, Athal Plant
Silvassa, Dadra & Nagar Haveli
Salary Package:
Salary will be best in the industry and commensurate with qualification, experience, and interview performance.
Walk-in Interview Details
Date: 08 February 2026
Time: 09:00 AM to 04:00 PM
Venue:
Hotel Sovereign,
Seaface Road, Nani Daman,
Daman – 396210
Documents to Carry
- Updated resume
- Passport size photograph
- ITI / Diploma / Graduation certificates
- 10th & 12th marksheets
- Experience letters and appointment letters
- Current CTC proof and recent payslips
Important Notes
- Only candidates with tablet and capsule manufacturing exposure should apply
- Candidates who attended interviews at IPCA Athal in the last six months should not apply
- MSC candidates will not be considered for QC department
- IPCA Laboratories does not charge any fees for recruitment
Why Join IPCA Laboratories Limited
- One of the world’s leading API and formulation manufacturers
- Strong exposure to USFDA and MHRA regulated manufacturing
- Structured career growth in QA, QC, and production
- Stable organization with long-term global presence
- Contribution to affordable and high-quality healthcare products
SEO-Optimized Job Titles
- IPCA Laboratories Athal Hiring ITI BPharm Candidates
- Pharma QA QC Production Jobs in Silvassa
- USFDA Approved Pharma Company Walk-in Interview
- Tablet Manufacturing Jobs at IPCA Laboratories
Frequently Asked Questions (FAQs)
Who can apply for IPCA Laboratories Athal walk-in interview?
Candidates with ITI, Diploma, B.Pharm, or B.Sc qualifications and relevant tablet manufacturing experience can apply.
Are freshers eligible for these vacancies?
Yes. Freshers are eligible for technician and trainee-level roles.
What manufacturing experience is required?
Only tablet and capsule manufacturing experience is considered for QA and QC roles.
Is shift duty mandatory?
Yes. Candidates must be willing to work in shift duties.
What is the interview venue?
The interview will be conducted at Hotel Sovereign, Nani Daman.
Summary Table
| Company | IPCA Laboratories Limited |
|---|---|
| Vacancies | Trainee/Officer – QA, Trainee/Officer – QC, Technical Supervisor – Production, Technician/Operator – Production & Packing, Technician – Engineering |
| Required Education | ITI, Diploma, B.Pharm, M.Pharm, B.Sc Chemistry |
| Experience | Fresher to 8 Years |