LYKA Labs Limited Hiring Multiple Pharma Professionals

- LYKA Labs Limited Hiring Multiple Pharma Professionals in Ankleshwar – Walk-In Interview 2026
- Company Overview
- Job Role & Responsibilities
- Quality Control – Executive (HPLC & GLP)
- Quality Control (Microbiology) – Officer / Assistant Manager
- Quality Assurance – Manager Validation / Senior Executive Validation
- Engineering Department – Water System & HVAC Engineers
- Production Department – HVAC Operator & Officers
- Eligibility / Qualifications
- Location & Salary
- Application Process
- Interview Details
- Required Documents
- Frequently Asked Questions (FAQs)
- Summary Table
LYKA Labs Limited Hiring Multiple Pharma Professionals in Ankleshwar – Walk-In Interview 2026
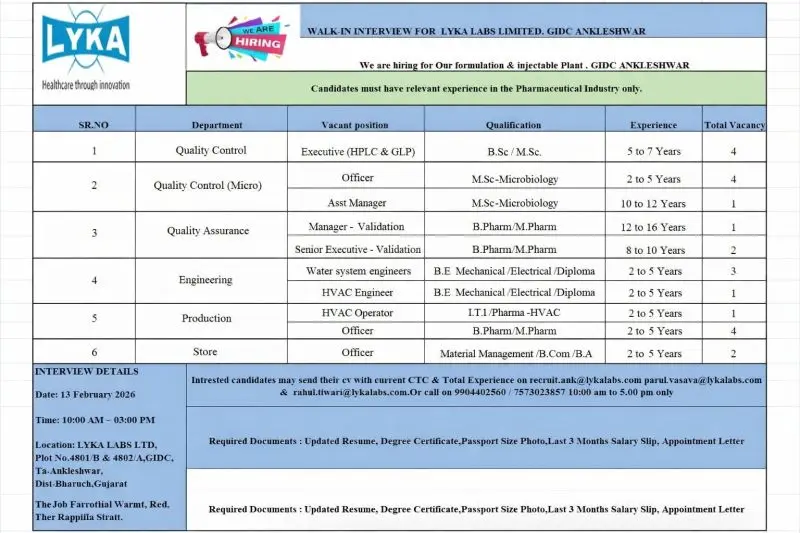
LYKA Labs Limited is conducting a walk-in interview to hire experienced pharmaceutical professionals across multiple departments at its formulation and injectable manufacturing plant in GIDC Ankleshwar, Gujarat. This hiring drive covers Quality Control, Quality Assurance, Engineering, Production, and Stores with a total of 23 open positions ranging from Officer and Executive roles to Assistant Manager and Manager-level positions. These vacancies are open only to candidates with prior experience in the pharmaceutical industry. The opportunity is based in Ankleshwar, Gujarat, one of India’s leading pharma manufacturing hubs.
This recruitment drive is ideal for professionals looking for stable pharma manufacturing jobs, GMP-regulated roles, validation careers, microbiology jobs, HPLC analyst positions, engineering roles in pharma plants, and production operations within a regulated injectable and formulation environment.
Company Overview
LYKA Labs Limited is a well-established pharmaceutical company with a strong legacy in manufacturing high-quality formulations and injectable products for domestic and international markets. The company operates with a deep focus on innovation, compliance, and patient safety while adhering strictly to global regulatory standards such as WHO-GMP, GLP, and cGMP.
With modern infrastructure at its Ankleshwar facility, LYKA Labs continues to strengthen its manufacturing capabilities across sterile and non-sterile dosage forms. The organization is known for maintaining robust quality systems, advanced analytical laboratories, validated manufacturing processes, and sustainable engineering practices. Working at LYKA Labs offers long-term career stability, exposure to regulated pharmaceutical manufacturing, and the opportunity to contribute directly to healthcare advancement through quality-driven operations.
Job Role & Responsibilities
Quality Control – Executive (HPLC & GLP)
Quality Control Executives will be responsible for analytical testing of raw materials, in-process samples, finished products, and stability samples using HPLC and other analytical instruments. The role requires strict adherence to GLP practices, documentation compliance, method execution, and data integrity.
Key responsibilities include:
- HPLC analysis and data review
- GLP documentation and compliance
- Calibration and maintenance of analytical instruments
- Handling OOS, deviations, and investigations
- Supporting regulatory audits and inspections
Total Vacancies: 4
Quality Control (Microbiology) – Officer / Assistant Manager
Microbiology professionals will manage microbiological testing for injectable and formulation products. Assistant Manager roles will also involve team handling, investigation management, and audit support.
Responsibilities include:
- Environmental monitoring and sterility testing
- Media preparation and microbial limit tests
- Water system microbiological analysis
- Aseptic area monitoring
- Investigation of microbial deviations
- Audit and regulatory compliance activities
Total Vacancies:
- Officer: 4
- Assistant Manager: 1
Quality Assurance – Manager Validation / Senior Executive Validation
Quality Assurance Validation professionals will be responsible for process validation, cleaning validation, equipment qualification, and system validation activities. These roles are critical for maintaining regulatory compliance and product quality.
Key responsibilities:
- Process, cleaning, and equipment validation
- Preparation and execution of validation protocols
- Risk assessment and change control
- Review of validation documents and reports
- Regulatory audit handling and compliance support
Total Vacancies:
- Manager Validation: 1
- Senior Executive Validation: 2
Engineering Department – Water System & HVAC Engineers
Engineering professionals will manage critical utility systems essential for pharmaceutical manufacturing. These roles ensure uninterrupted plant operations while maintaining GMP compliance.
Responsibilities include:
- Operation and maintenance of water systems (PW, WFI)
- HVAC system operation and validation support
- Preventive maintenance planning
- Documentation and compliance with engineering SOPs
- Support during regulatory inspections
Total Vacancies:
- Water System Engineers: 3
- HVAC Engineer: 1
Production Department – HVAC Operator & Officers
Production roles focus on manufacturing operations, equipment handling, and GMP-compliant production activities within formulation and injectable units.
Responsibilities include:
- Operation of HVAC systems in production areas
- Manufacturing operations as per SOPs and BMRs
- Material handling and documentation
- Compliance with GMP, safety, and quality standards
Total Vacancies:
- HVAC Operator: 1
- Production Officer (B.Pharm / M.Pharm): 4
- Stores Officer (Material Management): 2
Eligibility / Qualifications
Candidates must possess relevant pharmaceutical industry experience. Freshers are not eligible for this walk-in drive.
Required educational qualifications include:
B.Sc, M.Sc, M.Sc Microbiology, B.Pharm, M.Pharm, B.E Mechanical, B.E Electrical, Diploma in Mechanical Engineering, Diploma in Electrical Engineering, ITI, Pharma HVAC, B.Com, B.A
Experience requirements range from 2 to 16 years, depending on the role and seniority level.
Location & Salary
Job Location:
LYKA Labs Limited
Plot No. 4801 B & 4802/A, GIDC,
Taluka Ankleshwar,
District Bharuch, Gujarat
Application Process
This is a walk-in interview opportunity.
Interview Details
- Date: 13 February 2026
- Time: 10:00 AM to 03:00 PM
- Venue: LYKA Labs Limited, GIDC Ankleshwar, Gujarat
Required Documents
- Updated Resume
- Degree Certificates
- Passport Size Photographs
- Last 3 Months Salary Slips
- Appointment Letter / Experience Proof
Candidates unable to attend the walk-in may send their resume with current CTC and total experience to:
recruit.ank@lykalabs.com
parul.vasava@lykalabs.com
rahul.tiwari@lykalabs.com
Contact Numbers (10:00 AM to 5:00 PM only):
9904402560 | 7573023857
Frequently Asked Questions (FAQs)
Who can apply for LYKA Labs Ankleshwar vacancies?
Only candidates with relevant pharmaceutical industry experience are eligible.
Are freshers eligible for these roles?
No. This hiring drive is strictly for experienced professionals.
Is this a permanent position?
Yes. These are full-time permanent roles based at the Ankleshwar manufacturing facility.
Is prior injectable experience mandatory?
Injectable experience is preferred for relevant departments, especially QC Microbiology and QA Validation.
What type of interview will be conducted?
Face-to-face walk-in interviews with technical and HR panels.
Summary Table
| Category | Details |
|---|---|
| Company | LYKA Labs Limited |
| Vacancies | Quality Control Executive, QC Micro Officer, QC Micro Assistant Manager, QA Manager Validation, QA Senior Executive Validation, Water System Engineer, HVAC Engineer, HVAC Operator, Production Officer, Stores Officer |
| Required Education | B.Sc, M.Sc, M.Sc Microbiology, B.Pharm, M.Pharm, B.E Mechanical, B.E Electrical, Diploma, ITI, B.Com, B.A |
| Experience | 2 to 16 Years |
To apply for this job email your details to recruit.ank@lykalabs.com


