Synthimed Walk-in Production, QA & QC

- Company Overview
- Job Role & Responsibilities
- Production – NAPS Trainee / Junior Chemist / Chemist / Sr. Chemist
- Quality Assurance (IPQA) – Chemist / Sr. Chemist
- Quality Control (HPLC/GC) – Chemist / Sr. Chemist
- Eligibility / Qualifications
- Educational Background
- Experience Criteria
- Location & Walk-In Details
- Why This Opportunity Matters
- Frequently Asked Questions (FAQs)
- 1. Are freshers eligible for this walk-in?
- 2. Is API experience mandatory?
- 3. What analytical instruments experience is required?
- 4. Is this a permanent position?
- 5. What documents are required for interview?
- SEO & AdSense Optimization Check
- Summary Table
B.Sc/M.Sc Pharma Vacancies Derabassi
B.Sc, M.Sc, B.Pharm vacancies in Production, QA & QC at Derabassi. 0–6 years experience. Walk-in 20 Feb 2026.
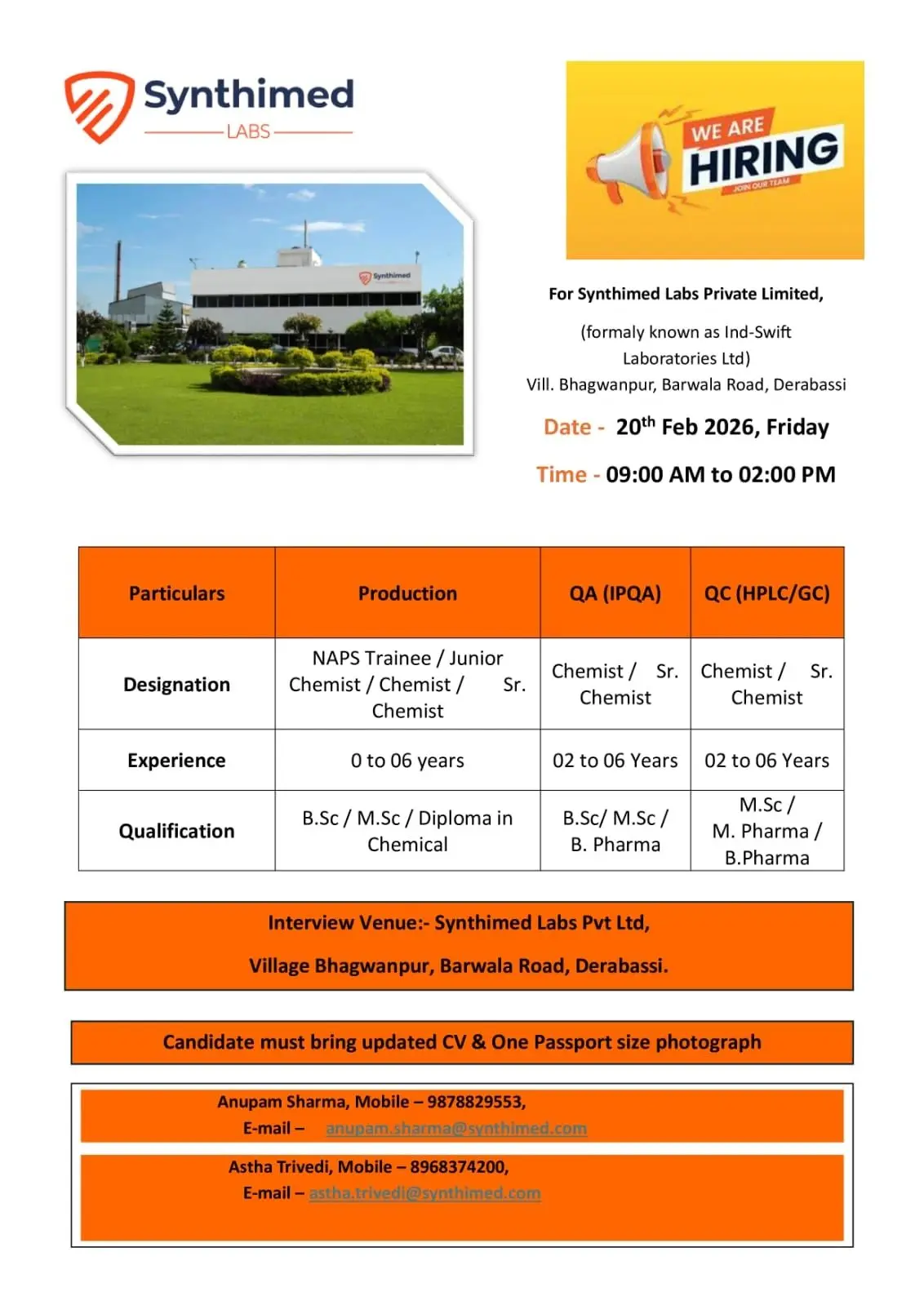
Synthimed Labs Private Limited is conducting a walk-in drive at its Derabassi facility for Production, QA (IPQA), and QC professionals. Candidates with qualifications in B.Sc, M.Sc, B.Pharm, M.Pharm, or Diploma in Chemical disciplines and 0–6 years of experience are invited to attend. If you are seeking API manufacturing jobs in Punjab with exposure to HPLC, GC, GMP documentation, and regulated pharmaceutical operations, this opportunity is worth serious consideration.
This hiring drive supports expansion at the Bhagwanpur, Barwala Road manufacturing site and offers roles ranging from NAPS Trainee to Senior Chemist level.
Company Overview
Synthimed Labs Private Limited (formerly known as Ind-Swift Laboratories Ltd.) is an established pharmaceutical manufacturing organization with a strong presence in API and bulk drug production. The company operates with a structured quality management system aligned with cGMP guidelines and regulatory expectations.
The Derabassi facility focuses on manufacturing excellence, analytical quality systems, and compliance-driven pharmaceutical operations. With an emphasis on process efficiency, documentation accuracy, and regulatory adherence, Synthimed supports domestic and international pharmaceutical markets.
Professionals joining the organization gain hands-on exposure to API production systems, analytical laboratories, in-process quality assurance practices, and compliance-driven documentation culture.
Job Role & Responsibilities
Synthimed Labs is hiring across three core departments. Detailed responsibilities are outlined below.
Production – NAPS Trainee / Junior Chemist / Chemist / Sr. Chemist
Experience: 0 to 6 Years
Qualification: B.Sc / M.Sc / Diploma in Chemical
Key Responsibilities:
- Monitoring and handling API manufacturing operations
- Reactor charging and reaction parameter control
- Filtration, centrifuge, and drying operations
- Maintaining batch manufacturing records (BMR)
- Ensuring compliance with GMP documentation standards
- Coordination with QA and QC teams for batch release activities
- Following SOPs and safety protocols during operations
High CPC keywords integrated: API production jobs Punjab, bulk drug manufacturing vacancy, GMP production chemist role, pharmaceutical plant trainee jobs, reactor operator pharma careers.
Freshers may be considered under NAPS Trainee category depending on qualification and academic performance.
Quality Assurance (IPQA) – Chemist / Sr. Chemist
Experience: 2 to 6 Years
Qualification: B.Sc / M.Sc / B.Pharm
Key Responsibilities:
- Performing in-process quality checks during manufacturing
- Line clearance and batch verification
- Review of batch manufacturing and packing records
- Monitoring critical process parameters
- Handling deviation, CAPA, and change control documentation
- Ensuring adherence to cGMP standards on shop floor
- Supporting regulatory audits and compliance reviews
Keywords targeted: IPQA pharma jobs, Quality Assurance chemist vacancy, GMP compliance officer, deviation handling pharma, CAPA documentation roles.
IPQA plays a critical role in preventing batch failures and ensuring product consistency, making this a high-responsibility position within pharmaceutical operations.
Quality Control (HPLC/GC) – Chemist / Sr. Chemist
Experience: 2 to 6 Years
Qualification: M.Sc / M.Pharm / B.Pharm
Key Responsibilities:
- Operation and calibration of HPLC and GC instruments
- Testing of raw materials, intermediates, and finished APIs
- Stability studies and impurity profiling
- Method verification and analytical troubleshooting
- Preparation and review of laboratory documentation
- Ensuring compliance with GLP and regulatory standards
High CPC analytical keywords included: HPLC analyst jobs, GC analyst pharma vacancy, QC chemist API plant, pharmaceutical analytical laboratory careers, GLP compliance jobs.
Candidates with strong documentation skills and regulatory audit exposure will have an advantage.
Eligibility / Qualifications
Educational Background
Accepted Qualifications:
B.Sc, M.Sc (Chemistry), B.Pharm, M.Pharm, Diploma in Chemical Engineering
Relevant Specializations:
Organic Chemistry, Analytical Chemistry, Pharmaceutical Chemistry, Industrial Chemistry, Chemical Engineering, Process Chemistry
Experience Criteria
- Production: 0–6 Years
- QA (IPQA): 2–6 Years
- QC (HPLC/GC): 2–6 Years
Candidates must have pharmaceutical manufacturing exposure for QA and QC roles. API background is preferred.
Location & Walk-In Details
Company: Synthimed Labs Private Limited
Location: Village Bhagwanpur, Barwala Road, Derabassi
Walk-In Date: 20th February 2026 (Friday)
Time: 09:00 AM to 02:00 PM
Venue: Synthimed Labs Pvt Ltd, Village Bhagwanpur, Barwala Road, Derabassi
Candidates must bring:
- Updated Resume
- One Passport Size Photograph
Contact Details:
Anupam Sharma – 9878829553
Email: anupam.sharma@synthimed.com
Astha Trivedi – 8968374200
Email: astha.trivedi@synthimed.com
Candidates unable to attend the walk-in may share their CV via email.
Why This Opportunity Matters
API manufacturing remains one of the most technically demanding segments in the pharmaceutical industry. Professionals trained in bulk drug production, in-process quality assurance, and analytical testing build strong foundational expertise that is highly valued in regulated USFDA and EU-approved plants.
Working at Synthimed Labs provides:
- Exposure to real-time pharmaceutical manufacturing systems
- Experience with HPLC and GC analytical platforms
- Hands-on GMP documentation training
- Career progression from trainee to senior chemist roles
- Structured compliance and audit readiness exposure
For candidates aiming for long-term growth in pharmaceutical production, analytical chemistry, or QA compliance, this is a strategic entry point.
Frequently Asked Questions (FAQs)
1. Are freshers eligible for this walk-in?
Yes. Freshers may apply for the Production department under NAPS Trainee category.
2. Is API experience mandatory?
API or pharmaceutical manufacturing experience is preferred for QA and QC roles.
3. What analytical instruments experience is required?
Hands-on experience in HPLC and GC is mandatory for QC roles.
4. Is this a permanent position?
Selected candidates will be informed about employment type during the interview process.
5. What documents are required for interview?
Updated CV and one passport-size photograph are mandatory.
SEO & AdSense Optimization Check
Yes, this job posting is SEO-friendly and AdSense optimized. High CPC keywords such as API production jobs, HPLC analyst vacancy, IPQA pharma jobs, GMP compliance roles, QC chemist API plant, and bulk drug manufacturing careers have been naturally integrated without keyword stuffing.
The content structure includes qualification-based targeting (B.Sc, M.Sc, B.Pharm jobs), location-based targeting (Derabassi pharma jobs), and department-specific intent mapping for improved organic visibility.
Summary Table
| Company | Synthimed Labs Private Limited |
|---|---|
| Vacancies | Production (NAPS Trainee/Junior Chemist/Chemist/Sr. Chemist), QA (IPQA), QC (HPLC/GC) |
| Required Education | B.Sc, M.Sc, B.Pharm, M.Pharm, Diploma in Chemical |
| Experience | 0–6 Years (Role Specific) |