Avenza Walk-In Analytical R&D, QC, QA, Packing, Production

- Company Overview
- Job Roles & Responsibilities
- Production Department – OSD Formulations
- Packing Department (Primary & Secondary)
- Quality Assurance Department
- Quality Control Department
- Analytical Research & Development (AR&D)
- Eligibility / Qualifications
- Location & Salary
- Walk-In Interview Details
- Application Process
- Frequently Asked Questions (FAQs)
- Summary Table
B.Pharm/M.Pharm Vacancies – Avenza Pharma Vadodara Walk-In
Avenza Pharmaceuticals hiring B.Pharm, M.Pharm, B.Sc, ITI candidates for Production, QA, QC & AR&D roles in Vadodara. Walk-in Dec 24–26.
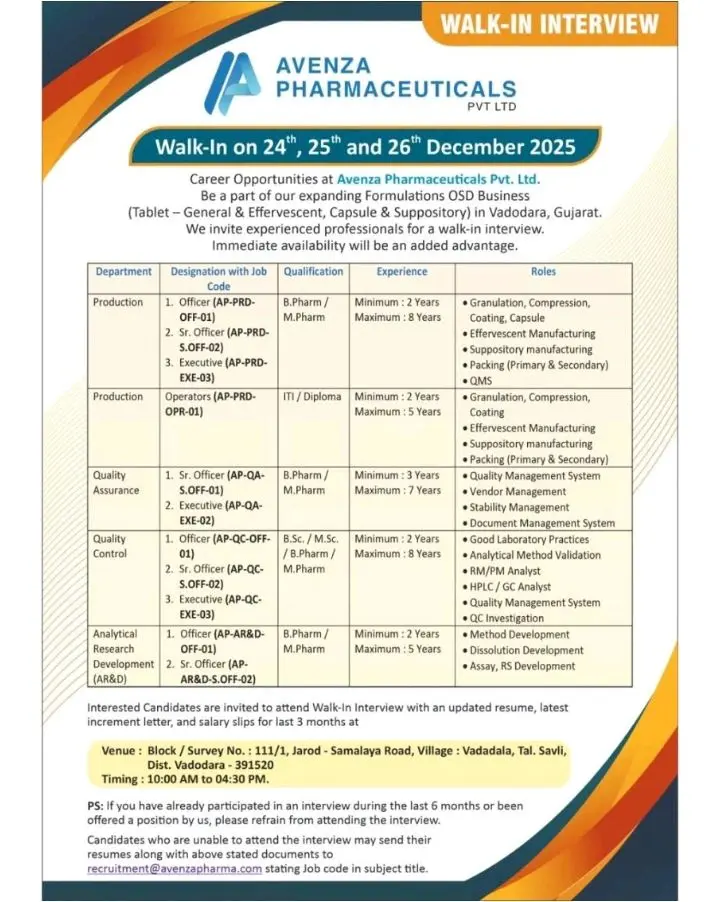
Avenza Pharmaceuticals Pvt. Ltd. is conducting a multi-day walk-in interview drive for experienced professionals to support its growing Formulations OSD manufacturing operations in Vadodara, Gujarat. This hiring initiative targets candidates with hands-on exposure to tablet, capsule, effervescent, and suppository dosage forms, offering stable career growth in a regulated pharmaceutical manufacturing environment. Candidates who can join immediately will be given preference.
Company Overview
Avenza Pharmaceuticals Pvt. Ltd. is a formulation-focused pharmaceutical company engaged in the manufacturing of oral solid dosage forms for domestic and international markets. The organization operates with strict adherence to cGMP, Quality Management Systems, and regulatory compliance standards, ensuring consistent product quality and patient safety. With expanding production capacities in Vadodara, Avenza continues to invest in skilled professionals across Production, Quality Assurance, Quality Control, and Analytical R&D functions.
Job Roles & Responsibilities
Production Department – OSD Formulations
Designations: Officer, Senior Officer, Executive
Key Responsibilities:
- Handling OSD manufacturing activities including granulation, compression, coating, capsule filling, effervescent manufacturing, and suppository production
- Operation and monitoring of manufacturing equipment as per SOPs
- Compliance with cGMP, safety, and documentation requirements
- Execution of batch manufacturing records and OMS documentation
Production Operators (ITI/Diploma):
- Operation of granulation, compression, coating, and packing equipment
- Primary and secondary packing activities
Packing Department (Primary & Secondary)
- Blister and bottle packing operations
- Line clearance, in-process checks, and packing documentation
- OMS compliance and coordination with QA
Quality Assurance Department
Designations: Senior Officer, Executive
Key Responsibilities:
- Implementation and maintenance of Quality Management Systems (QMS)
- Handling deviations, change controls, CAPA, and investigations
- Review of batch records, SOPs, and compliance documentation
Quality Control Department
Designations: Officer, Senior Officer, Executive
Key Responsibilities:
- Analysis of raw materials, finished products, and stability samples
- Operation of HPLC, GC, dissolution, and wet chemistry techniques
- Method validation, investigation handling, and documentation
- Compliance with GLP and data integrity guidelines
Analytical Research & Development (AR&D)
Designations: Officer, Senior Officer
Key Responsibilities:
- Analytical method development and validation
- Dissolution development, assay development, and RS development
- Stability management and vendor-related analytical activities
- Support to product development and regulatory submissions
Eligibility / Qualifications
Required Education (role-specific):
B.Pharm, M.Pharm, B.Sc, M.Sc, ITI, Diploma
Relevant Courses:
B.Pharmacy, M.Pharmacy, B.Sc Chemistry, M.Sc Chemistry, Pharmaceutical Analysis, Industrial Pharmacy, Quality Assurance, Analytical Chemistry
Experience Range:
- Production Officers/Executives: 2–8 years
- Operators: 2–5 years
- QA Roles: Minimum 3 years
- QC Roles: 2–8 years
- AR&D Roles: 2–7 years
Location & Salary
Work Location: Vadodara, Gujarat
Salary will be industry-aligned and commensurate with experience, along with structured growth opportunities in a formulation-focused organization.
Walk-In Interview Details
Dates: 24th, 25th & 26th December 2025
Time: 10:00 AM to 04:30 PM
Venue: Block/Survey No. 111/1, Jarod–Samalaya Road, Village Vadadala, Tal. Savli, Dist. Vadodara – 391520
Application Process
Interested candidates should attend the walk-in interview with:
- Updated resume
- Latest increment letter
- Salary slips for the last 3 months
Candidates unable to attend may email their resume and documents to:
recruitment@avenzapharma.com
(Kindly mention the Job Code in the email subject line.)
Note: Candidates interviewed or offered a role within the last 6 months should not apply.
Frequently Asked Questions (FAQs)
Q1. Which departments are hiring at Avenza Pharmaceuticals?
Production, Packing, Quality Assurance, Quality Control, and Analytical R&D.
Q2. Is formulation experience mandatory?
Yes. Only candidates with OSD formulations experience are eligible.
Q3. Can freshers apply for this walk-in drive?
No. This drive is strictly for experienced professionals.
Q4. What analytical instruments experience is required for QC roles?
Hands-on exposure to HPLC, GC, dissolution, and wet chemistry is preferred.
Summary Table
| Company | Avenza Pharmaceuticals Pvt. Ltd. |
|---|---|
| Vacancies | Production, QA, QC, AR&D, Packing |
| Required Education | B.Pharm, M.Pharm, B.Sc, M.Sc, ITI, Diploma |
| Experience | 2 to 8 Years (role dependent) |