Concord walk-in Production, QA, QC, RA & ADL
- Company Overview
- Job Roles & Responsibilities
- Production & Packaging (OSD / Injectables)
- Quality Control & Microbiology
- Quality Assurance (IPQA & Qualification)
- Regulatory Affairs (Global & Domestic)
- Analytical Development Laboratory (Injectables)
- Eligibility / Qualifications
- Location & Work Sites
- Walk-In Schedule
- Application Process
- FAQs
- Summary Table
B.Pharm/M.Pharm Walk-In – Concord Biotech Ahmedabad
Concord Biotech walk-in interview Ahmedabad for Production, QA, QC, RA & ADL roles. B.Pharm, M.Pharm, BSc, MSc eligible.
Concord Biotech Limited has announced a major walk-in interview drive at its Ahmedabad manufacturing units for experienced and early-career professionals across Production, Packaging, Quality Assurance, Quality Control, Regulatory Affairs, and Analytical Development Laboratory (Injectables). This hiring initiative targets candidates with hands-on exposure to regulated pharmaceutical manufacturing, sterile injectables, and global regulatory operations. The walk-in drive provides a direct opportunity to join one of India’s most respected biopharmaceutical companies with strong expertise in injectables, fermentation-based APIs, and complex drug delivery systems.
Company Overview
Concord Biotech Limited is a globally recognized biopharmaceutical manufacturer with a strong presence in fermentation-based APIs, sterile injectables, and finished dosage formulations. The company supplies regulated markets including the US, Europe, and other highly compliant regions, operating under stringent USFDA, EU-GMP, and WHO-GMP standards.
With state-of-the-art facilities in Ahmedabad, Concord Biotech is known for its robust quality systems, advanced manufacturing technologies, and commitment to patient-centric healthcare solutions. Careers at Concord Biotech offer long-term stability, exposure to global regulatory environments, and structured growth across technical and leadership roles.
Job Roles & Responsibilities
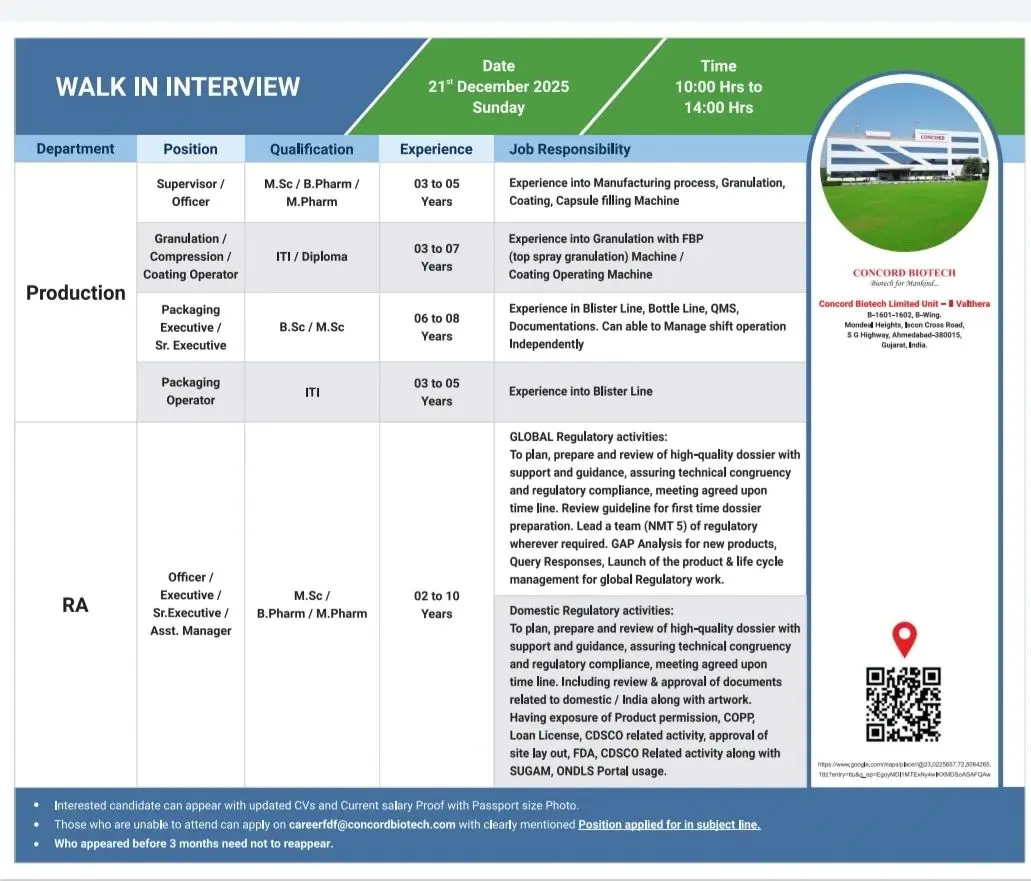
Production & Packaging (OSD / Injectables)
- Operation and supervision of Granulation, Compression, Coating, and Capsule Filling machines
- Hands-on experience with FBP Top Spray Granulation, Coating Machines, and high-speed equipment
- Execution of Blister and Bottle Packing operations with full documentation
- Independent shift handling, adherence to cGMP, and batch manufacturing record compliance
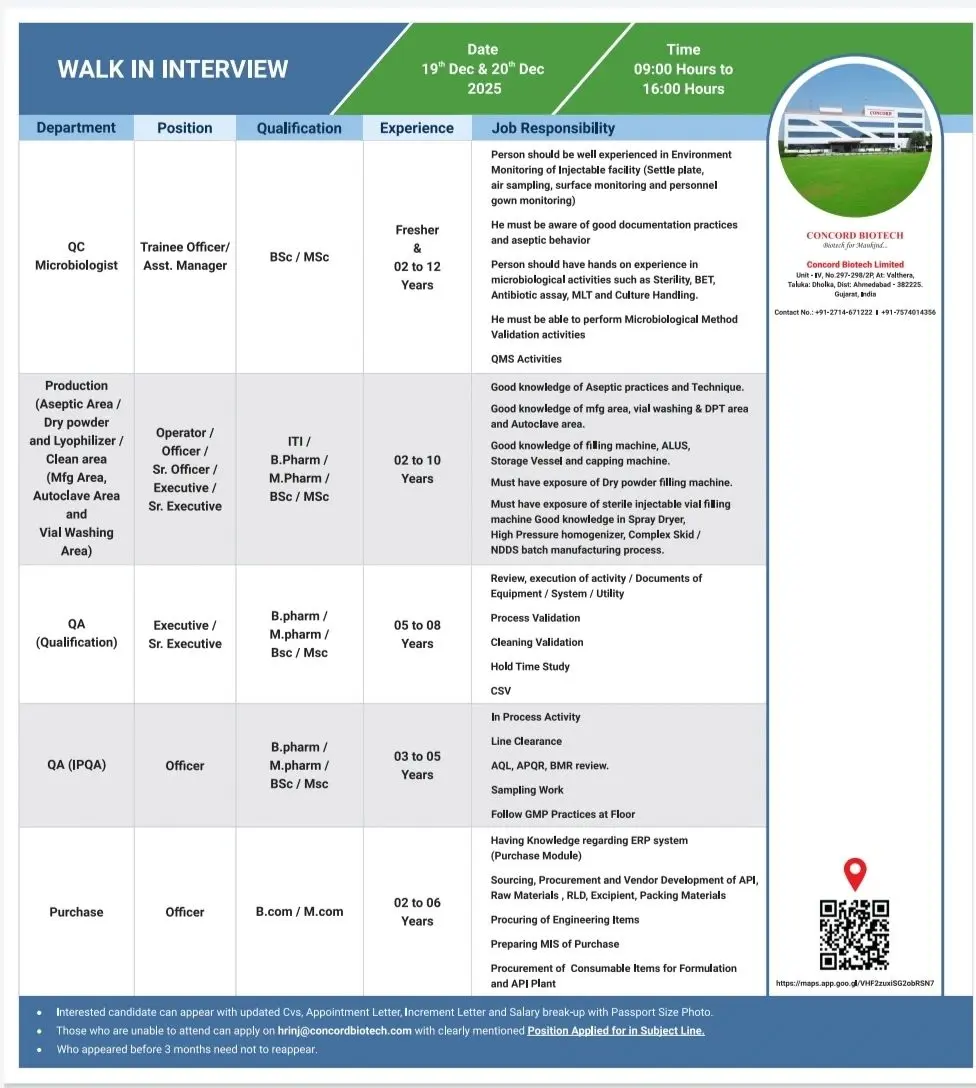
Quality Control & Microbiology
- Environmental Monitoring of injectable facilities including settle plates, air sampling, surface and personnel monitoring
- Sterility testing, BET, MLT, antibiotic assay, and culture handling
- Microbiological method validation and routine QC analysis
- Compliance with GLP, data integrity, and laboratory QMS requirements
Quality Assurance (IPQA & Qualification)
- On-floor IPQA activities across aseptic manufacturing areas
- Line clearance, in-process checks, AQL, APQR, and BMR review
- Process validation, cleaning validation, CSV, and hold time studies
- Review and execution of equipment, system, and utility qualification documentation
Regulatory Affairs (Global & Domestic)
- Preparation, review, and submission of high-quality regulatory dossiers
- Global regulatory lifecycle management including variations, query responses, and product launches
- Domestic regulatory submissions, artwork approvals, COPP, loan licenses, and CDSCO activities
- Hands-on experience with SUGAM and ONDLS portals
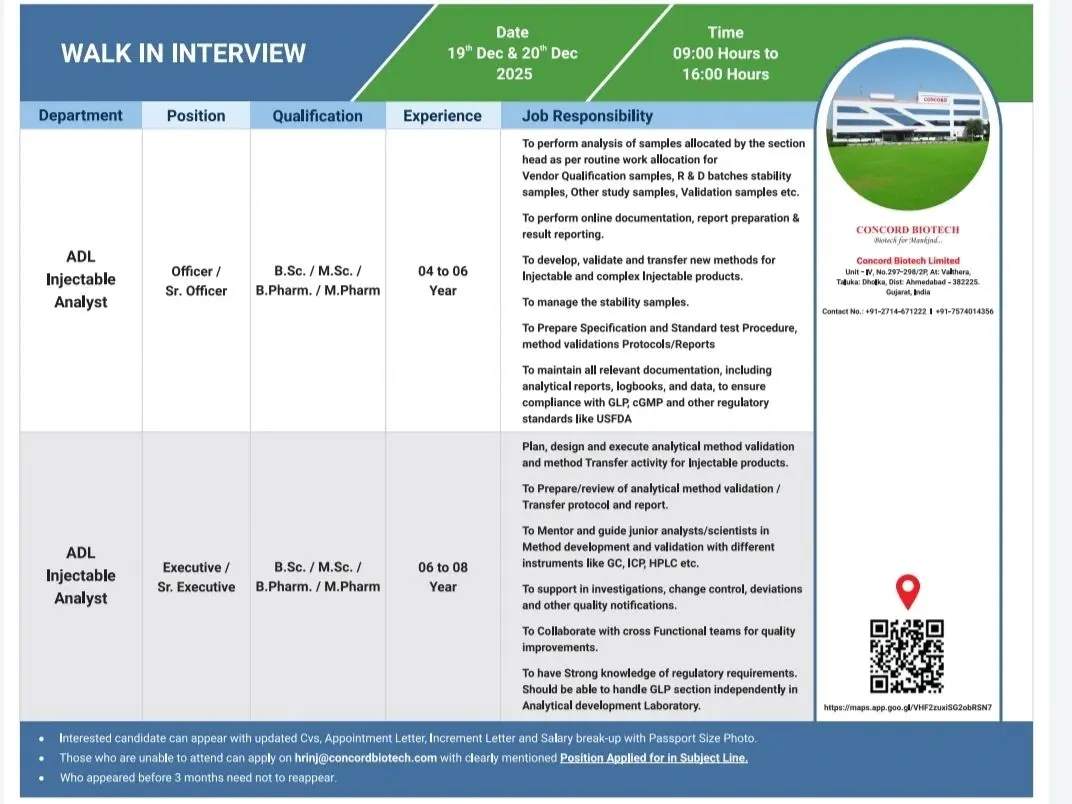
Analytical Development Laboratory (Injectables)
- Analysis of validation, stability, R&D, and vendor qualification samples
- Development, validation, and transfer of analytical methods for injectable products
- Preparation of STPs, specifications, validation protocols, and reports
- Strong exposure to HPLC, GC, ICP, and advanced analytical instruments
- Support investigations, deviations, change control, and quality improvements
Eligibility / Qualifications
Required Education (role-dependent):
B.Pharm, M.Pharm, B.Sc, M.Sc, ITI, Diploma Engineering, B.Com, M.Com
Experience Range:
Freshers to 12 years depending on department and designation
Location & Work Sites
- Ahmedabad, Gujarat
- Concord Biotech Limited – Unit IV & Unit V (Valthers / Dholka)
Walk-In Schedule
- Dates: 19th & 20th December 2025, and 21st December 2025
- Time: 09:00 AM to 04:00 PM (role-specific timing applies)
- Interview Mode: Direct walk-in
Application Process
Eligible candidates may attend the walk-in interview with:
- Updated CV
- Passport size photograph
- Appointment letter, increment letter, and salary breakup
Candidates unable to attend may email their CVs with the position applied for in the subject line:
- Production / RA: careerfdf@concordbiotech.com
- Injectables / QA / QC / ADL: hrinj@concordbiotech.com
Candidates who have appeared for an interview in the last three months need not reapply.
FAQs
Is this walk-in open for freshers?
Yes. Selected QC Microbiology and Production roles are open to freshers.
Are regulatory affairs roles included?
Yes. Both global and domestic regulatory affairs positions are part of this drive.
Is injectable experience mandatory for all roles?
Injectable experience is mandatory for QA, QC Microbiology, and ADL roles.
What type of company is Concord Biotech?
Concord Biotech is a leading biopharmaceutical company specializing in fermentation APIs and sterile injectables.
Summary Table
Company Concord Biotech Limited
Vacancies Multiple
Required Education B.Pharm, M.Pharm, B.Sc, M.Sc, ITI, Diploma, B.Com, M.Com
Experience Fresher to 12 Years