Lupin Fresher Hiring QC & API Production Vacancies

- QC & API Production Vacancies – Lupin Mandideep
- Company Overview
- Job Role & Responsibilities
- Designations Offered
- Quality Control (API / FML) Responsibilities
- Production – API Responsibilities
- Eligibility / Qualifications
- Educational Qualifications
- Experience Requirements
- Additional Eligibility Conditions
- Location & Salary
- Job Location
- Walk-in Interview Details
- Documents Required (Photocopies)
- Application Process
- Frequently Asked Questions (FAQs)
- Who can attend this Lupin walk-in interview?
- Are freshers eligible?
- Is shift work mandatory?
- What departments are hiring?
QC & API Production Vacancies – Lupin Mandideep
Lupin Ltd walk-in interview at Mandideep, Bhopal for QC and API Production roles. B.Pharm, Diploma, BSc, MSc. Multiple vacancies.
Lupin Ltd has announced a walk-in interview for experienced and entry-level professionals at its Mandideep manufacturing site near Bhopal, Madhya Pradesh. This hiring drive is focused on strengthening Quality Control and API Production teams across multiple designations. The opportunity is ideal for candidates with pharmaceutical manufacturing exposure who want to work with one of India’s most respected global pharma companies in a regulated, technology-driven environment.
The Mandideep facility plays a key role in Lupin’s API and formulation manufacturing operations. With increasing demand for high-quality active pharmaceutical ingredients and finished dosage forms, Lupin is expanding its workforce to support compliance, productivity, and operational excellence.
Company Overview
Lupin Ltd is a globally recognized pharmaceutical company with a strong presence across APIs, formulations, and specialty products. Headquartered in India, Lupin operates manufacturing facilities that comply with global regulatory standards including USFDA, EMA, MHRA, and WHO-GMP.
The Mandideep site near Bhopal is a critical manufacturing hub supporting API and formulation manufacturing lines. The facility follows strict cGMP norms, data integrity standards, and quality systems. Lupin’s consistent focus on research, quality, and compliance has positioned it as a trusted supplier of affordable medicines across domestic and international markets.
Working at Lupin provides professionals with long-term stability, exposure to regulated markets, and opportunities to build strong technical expertise in pharmaceutical manufacturing, quality systems, and production operations.
Job Role & Responsibilities
Lupin is hiring for multiple designations across Quality Control and Production (API) departments. Selected candidates will be responsible for ensuring quality compliance, smooth production operations, and adherence to regulatory standards.
Designations Offered
- EA – Associate
- EO – Junior Officer
- E1 – Officer
Quality Control (API / FML) Responsibilities
- Sampling and testing of raw materials and excipients
- Analysis using HPLC, GC, IR, UV, Dissolution, Polarimeter, KF, PSD (Malvern)
- Support stability studies and documentation
- Ensure compliance with data integrity and cGMP requirements
- Handle OOS, OOT, deviations, and QMS activities (CAPA, CCP)
- Maintain laboratory records and work on LIMS software
- Follow SOPs and regulatory guidelines strictly
Production – API Responsibilities
- Execute shift-based production shop-floor activities
- Processing of API batches as per approved procedures
- Online filing of BPRs and production records
- Perform SAP-related documentation activities
- Operate reactors, sparkler filters, candle filters, centrifuges, dryers
- Ensure equipment operation as per SOP and safety guidelines
- Maintain communication with cross-functional teams
Good oral and written communication skills are essential for both roles.
Eligibility / Qualifications
Educational Qualifications
- Quality Control: B.Pharmacy
- Production – API: Diploma (Chemical / Mechanical), BSc, MSc – Chemistry
Relevant courses include:
B.Pharmacy, Diploma in Chemical Engineering, Diploma in Mechanical Engineering, BSc Chemistry, MSc Chemistry
Experience Requirements
- Quality Control: 2 to 7 years
- Production – API: Freshers to 7 years
Candidates with less than 5 years of experience must have a minimum of 60% marks in their highest qualification.
Additional Eligibility Conditions
- Candidates interviewed within the last 6 months are not eligible
- Willingness to work in shifts is mandatory
- Strong understanding of pharmaceutical manufacturing environments preferred
Location & Salary
Job Location
- Mandideep (Bhopal), Madhya Pradesh
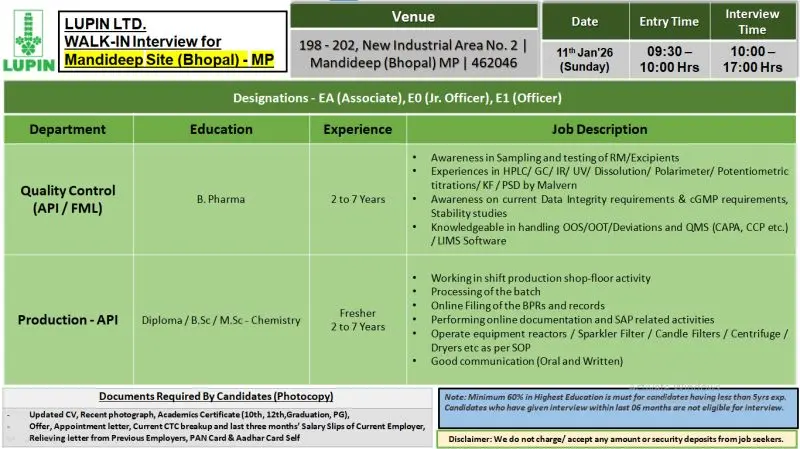
Walk-in Interview Details
Date: 11 January 2026 (Sunday)
Entry Time: 09:30 AM – 10:00 AM
Interview Time: 10:00 AM – 05:00 PM
Venue:
Lupin Ltd
198–202, New Industrial Area No. 2
Mandideep (Bhopal), Madhya Pradesh – 462046
Candidates are advised to report within the entry time window for smooth registration.
Documents Required (Photocopies)
- Updated CV
- Recent passport-size photograph
- Academic certificates (10th, 12th, Graduation, PG)
- Current offer or appointment letter
- Current CTC breakup and last three months’ salary slips
- Relieving letters from previous employers
- PAN Card and Aadhaar Card
Application Process
This is a direct walk-in interview. Eligible candidates should walk in directly to the venue with the required documents on the mentioned date.
Lupin Ltd does not charge or accept any fees or security deposits from job seekers at any stage of the hiring process.
Frequently Asked Questions (FAQs)
Who can attend this Lupin walk-in interview?
Candidates with relevant qualifications and experience in QC or API Production who meet the eligibility criteria can attend.
Are freshers eligible?
Freshers are eligible only for Production – API roles. QC roles require prior experience.
Is shift work mandatory?
Yes. Production roles require shift-based work.
What departments are hiring?
Quality Control (API & FML) and Production (API).
| Category | Details |
|---|---|
| Company | Lupin Ltd |
| Vacancies | Multiple (EA, EO, E1) |
| Required Education | B.Pharm, Diploma, BSc, MSc Chemistry |
| Experience | Fresher to 7 Years |


