MSN Walk-in QC, QA & Microbiology

- Company Overview
- Job Role & Responsibilities
- Quality Control Department
- Microbiology Department
- Quality Assurance Department
- Injectables & Packing Operations
- Eligibility / Qualifications
- Required Education
- Required Skills
- Location & Salary
- Documents Required for Walk-in
- Why Build a Career at MSN Laboratories
- Application Process
- Frequently Asked Questions (FAQs)
- Is this walk-in only for experienced candidates?
- Where is the job location?
- Is injectable experience mandatory?
- What departments are hiring?
- Career Growth at MSN Laboratories
- Summary Table
B.Pharm, MSc QC QA Jobs at MSN Vizag Walk-in
MSN Laboratories hiring QC, QA & Microbiology professionals in Vizag. B.Pharm, MSc eligible. 2–8 years experience.
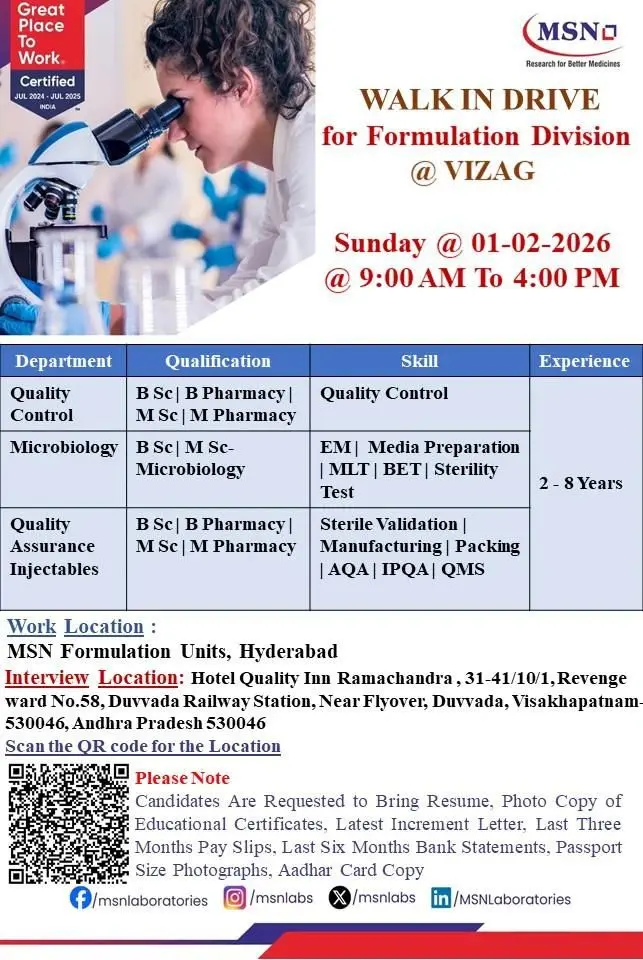
MSN Laboratories, a Great Place to Work–certified pharmaceutical organization, is conducting a walk-in drive for its Formulation Division to strengthen Quality Control, Quality Assurance, Microbiology, Injectables, and Packing operations. This recruitment drive is aimed at professionals with hands-on experience in regulated pharmaceutical manufacturing who want to contribute to high-quality injectable and formulation products supplied to global markets.
The walk-in interview will be held in Visakhapatnam, while the work location will be at MSN’s formulation units in Hyderabad. Candidates with strong GMP exposure and a compliance-focused mindset are encouraged to attend.
Company Overview
MSN Laboratories is one of India’s fastest-growing pharmaceutical companies with a strong presence in regulated markets such as the US, Europe, and other international regions. The organization is widely recognized for its research-driven approach, robust quality systems, and consistent regulatory compliance.
Certified as a Great Place to Work, MSN Laboratories promotes a culture of innovation, accountability, and continuous improvement. Its formulation and injectable facilities operate under stringent cGMP guidelines, supporting the development and manufacturing of affordable, high-quality medicines that improve patient outcomes globally.
Job Role & Responsibilities
MSN Laboratories is hiring experienced professionals across multiple quality and manufacturing functions within its Formulation Division.
Quality Control Department
Qualification: B.Sc, B.Pharm, M.Sc, M.Pharm
Experience: 2 to 8 Years
Key Responsibilities
- Analysis of raw materials, in-process samples, and finished products
- Media preparation and microbiological testing support
- Performing MLT, BET, and sterility testing activities
- Maintaining laboratory documentation in compliance with GMP and data integrity guidelines
- Supporting investigations, deviations, and audit readiness
Microbiology Department
Qualification: B.Sc / M.Sc – Microbiology
Experience: 2 to 8 Years
Key Responsibilities
- Environmental monitoring of cleanroom areas
- Media preparation, sterility testing, and microbial limit testing
- Supporting microbiological validation activities
- Ensuring compliance with GMP and aseptic practices
Quality Assurance Department
Qualification: B.Sc, B.Pharm, M.Sc, M.Pharm
Experience: 2 to 8 Years
Key Responsibilities
- Handling sterile validation and qualification activities
- Managing manufacturing QA, AQA, IPQA, and QMS functions
- Reviewing batch records, deviations, and CAPA
- Supporting internal audits and regulatory inspections
Injectables & Packing Operations
Qualification: B.Sc, B.Pharm, M.Sc, M.Pharm
Experience: 2 to 8 Years
Key Responsibilities
- Supporting injectable manufacturing and packing operations
- Ensuring adherence to aseptic processing and SOP requirements
- Maintaining BMR, logbooks, and compliance documentation
- Coordinating with QA and QC teams during routine operations
Eligibility / Qualifications
Required Education
B.Sc, B.Pharm, M.Sc, M.Pharm, B.Sc/M.Sc in Microbiology
Required Skills
- Strong understanding of cGMP and data integrity principles
- Hands-on experience in sterile manufacturing environments
- Knowledge of MLT, BET, sterility testing, and environmental monitoring
- Familiarity with QA systems such as IPQA, AQA, and QMS
Location & Salary
Work Location: MSN Formulation Units, Hyderabad
Interview Location:
Hotel Quality Inn Ramachandra,
31-41/10/1, Revenue Ward No.58,
Near Duvvada Railway Station Flyover,
Duvvada, Visakhapatnam – 530046,
Andhra Pradesh, India
Date & Time:
Sunday, 01 February 2026 | 9:00 AM to 4:00 PM
Salary:
Salary will be offered as per industry standards and will be commensurate with experience, role, and regulatory exposure.
Documents Required for Walk-in
Candidates are requested to carry the following documents:
- Updated resume
- Photocopies of educational certificates
- Latest increment letter
- Last three months’ salary slips
- Last six months’ bank statements
- Passport-size photographs
- Aadhaar card copy
Why Build a Career at MSN Laboratories
Careers in injectable manufacturing and pharmaceutical quality functions attract high CPC search demand due to regulatory complexity and global compliance requirements. Keywords such as QC QA jobs in pharma, injectable manufacturing jobs, microbiology jobs in pharmaceutical companies, and GMP compliance careers are among the most searched roles in the industry.
At MSN Laboratories, professionals gain exposure to regulated injectable facilities, robust quality systems, and global audit environments while contributing directly to life-saving therapies.
Application Process
Interested candidates can directly attend the walk-in interview at the specified venue and date.
For updates and employer information, candidates may follow MSN Laboratories on official social channels.
Frequently Asked Questions (FAQs)
Is this walk-in only for experienced candidates?
Yes. This walk-in drive is open only to candidates with 2–8 years of relevant pharmaceutical experience.
Where is the job location?
The job location is at MSN Laboratories’ Formulation Units in Hyderabad.
Is injectable experience mandatory?
Injectable and sterile manufacturing experience is strongly preferred for most roles.
What departments are hiring?
Quality Control, Quality Assurance, Microbiology, Injectables, and Packing departments.
Career Growth at MSN Laboratories
Working in a Great Place to Work–certified organization provides long-term career stability, skill development, and leadership opportunities. Quality and injectable manufacturing professionals at MSN Laboratories gain high-value regulatory exposure that supports global career mobility.
By joining MSN Laboratories, candidates become part of a mission that delivers affordable, high-quality medicines to patients worldwide.
Summary Table
| Category | Details |
|---|---|
| Company | MSN Laboratories |
| Vacancies | Quality Control Executive, Microbiology Executive, Quality Assurance Executive, Injectables & Packing Professionals |
| Required Education | B.Sc, B.Pharm, M.Sc, M.Pharm, B.Sc/M.Sc Microbiology |
| Experience | 2–8 Years |