Pfizer Walk-In manufacturing

- Company Overview
- Job Role & Responsibilities
- Manufacturing – Experienced Operators (Injectables)
- Manufacturing Compliance Roles (QMS)
- Manufacturing – Supervisors (Injectables)
- Senior Equipment Specialist (Visual Inspection)
- Eligibility / Qualifications
- Required Education
- Experience Requirements
- Location & Salary
- Application Process
- Frequently Asked Questions (FAQs)
- Summary Table
Pfizer Walk-In Interview – Diploma/BSc/MPharm – Vapi
Pfizer hiring manufacturing professionals at Vapi. Diploma, BSc, BPharm, MPharm with 2–12 years experience. Walk-in interview Jan 4, 2026.
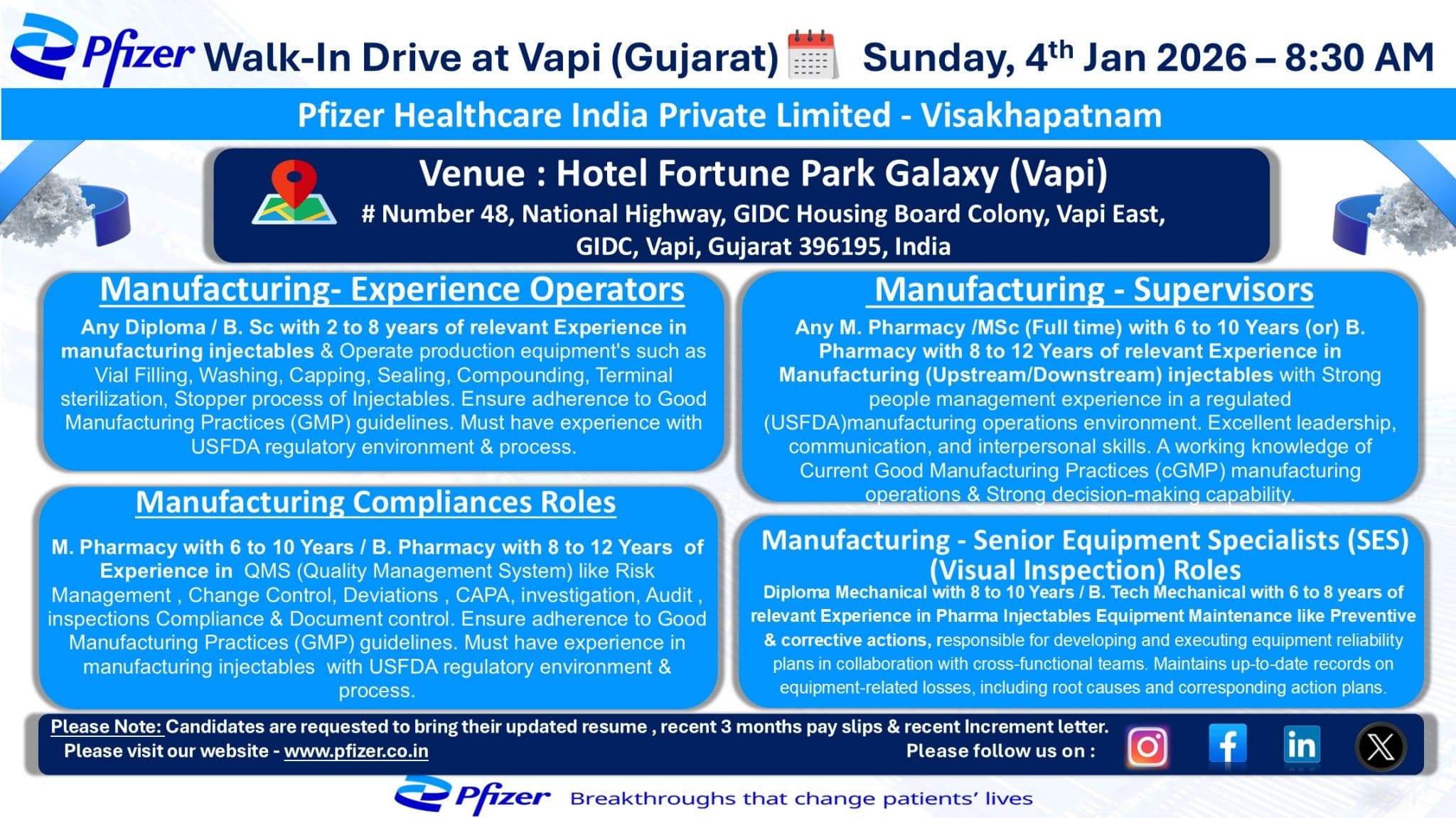
Pfizer Healthcare India Private Limited has announced a walk-in interview drive for experienced pharmaceutical manufacturing professionals at Vapi, Gujarat. This hiring initiative targets skilled candidates with hands-on exposure in injectable manufacturing, quality compliance, equipment maintenance, and regulated USFDA manufacturing environments. The drive offers a strong opportunity to join a globally respected pharmaceutical company known for innovation, compliance excellence, and long-term career growth.
This walk-in drive is ideal for professionals looking to build or advance their careers in sterile injectables, manufacturing compliance, equipment reliability, and people leadership roles within a highly regulated global pharma setup.
Company Overview
Pfizer is one of the world’s leading biopharmaceutical companies, committed to discovering, developing, and delivering breakthrough medicines that improve patient lives globally. With a strong presence in India, Pfizer Healthcare India Private Limited operates world-class manufacturing and compliance-driven facilities aligned with USFDA, EMA, and global cGMP standards.
Careers at Pfizer offer exposure to advanced technologies, robust quality systems, and a culture driven by integrity, innovation, and patient-centricity. Working at Pfizer means being part of a global organization that sets benchmarks in pharmaceutical manufacturing and regulatory compliance.
Job Role & Responsibilities
Manufacturing – Experienced Operators (Injectables)
- Operate injectable manufacturing equipment including vial washing, filling, capping, sealing, compounding, terminal sterilization, and stopper processing
- Execute manufacturing activities in compliance with cGMP and USFDA regulatory requirements
- Maintain batch manufacturing records, logbooks, and production documentation
- Ensure adherence to safety, quality, and data integrity practices
Manufacturing Compliance Roles (QMS)
- Manage Quality Management System activities including deviations, CAPA, change control, investigations, and risk management
- Support internal and external audits, regulatory inspections, and compliance documentation
- Ensure document control and alignment with global GMP standards
- Collaborate with manufacturing teams to drive compliance excellence
Manufacturing – Supervisors (Injectables)
- Lead upstream and downstream injectable manufacturing operations
- Manage shop-floor teams, shift planning, and production targets
- Ensure compliance with cGMP and USFDA regulations
- Drive continuous improvement, people development, and operational excellence
Senior Equipment Specialist (Visual Inspection)
- Perform preventive and corrective maintenance of injectable manufacturing equipment
- Develop and execute equipment reliability and maintenance plans
- Analyze equipment losses, perform root cause analysis, and implement corrective actions
- Maintain accurate equipment documentation and compliance records
Eligibility / Qualifications
Required Education
Diploma in Mechanical Engineering, Diploma (Any Discipline), B.Sc, B.Pharm, M.Pharm, M.Sc (Full-time)
Experience Requirements
- Manufacturing Operators: 2 to 8 years in injectable manufacturing
- Manufacturing Compliance (QMS): M.Pharm with 6–10 years or B.Pharm with 8–12 years
- Manufacturing Supervisors: M.Pharm/M.Sc with 6–10 years or B.Pharm with 8–12 years
- Senior Equipment Specialist: Diploma Mechanical with 8–10 years or B.Tech Mechanical with 6–8 years
Mandatory experience in regulated pharmaceutical manufacturing environments with strong exposure to USFDA standards.
Location & Salary
- Job Location: Vapi, Gujarat
- Interview Venue: Hotel Fortune Park Galaxy, NH-48, GIDC Housing Board Colony, Vapi East, Gujarat – 396195
- Salary: Attractive and industry-aligned compensation based on role, experience, and skill set
Application Process
This is a direct walk-in interview.
Interview Date: Sunday, 4th January 2026
Reporting Time: 8:30 AM
Candidates must carry:
- Updated resume
- Last 3 months salary slips
- Latest increment letter
For more details, visit the official website: https://www.pfizer.co.in
Frequently Asked Questions (FAQs)
Who can attend this Pfizer walk-in interview?
Candidates with Diploma, B.Sc, B.Pharm, M.Pharm, or M.Sc qualifications and relevant experience in injectable manufacturing or compliance can attend.
Is USFDA experience mandatory?
Yes. Prior experience in USFDA-regulated manufacturing environments is required.
Is this a permanent role?
Yes. These are full-time roles within Pfizer’s manufacturing operations.
Is this walk-in open for freshers?
No. This drive is for experienced professionals only.
Summary Table
| Company | Pfizer Healthcare India Private Limited |
|---|---|
| Vacancies | Multiple |
| Required Education | Diploma, B.Sc, B.Pharm, M.Pharm, M.Sc |
| Experience | 2 to 12 years depending on role |