Stellar Formulations Walk-in Production, QA, QC, Engineering

- Company Overview
- Job Role & Responsibilities
- Production
- Warehouse
- Engineering
- Quality Control (QC)
- Quality Assurance (QA)
- Documentation & Other Areas
- Formulation & Development (F&D)
- Eligibility / Qualifications
- Required Education
- Experience Requirement
- Location & Salary
- Interview Details
- Application Process
- Why This Opportunity Matters
- Frequently Asked Questions (FAQs)
- Is OSD formulation experience mandatory?
- Can freshers apply?
- What qualifications are accepted?
- Is this a direct company hiring?
- Summary Table
OSD Pharma Jobs – B.Pharm/M.Pharm – Savli
Stellar Formulations hiring Production, QA, QC, Engineering at Savli, Vadodara. OSD experience required. Walk-in Jan 2026.
Stellar Formulations Industries Private Limited is conducting walk-in interviews at its Savli (Vadodara) manufacturing unit for multiple positions across Production, Quality, Engineering, Warehouse, and Formulation & Development departments. This hiring drive targets experienced pharmaceutical professionals with solid oral dosage (OSD) formulation exposure, offering an opportunity to work in a quality-driven, growth-oriented pharmaceutical manufacturing organization.
Candidates with hands-on experience in OSD formulations who are seeking stable roles, technical growth, and long-term career progression in pharmaceutical manufacturing are encouraged to attend.
Company Overview
Stellar Formulations Industries Pvt Ltd is a pharmaceutical company specializing in the manufacturing and supply of solid oral dosage formulations. The company is committed to delivering high-quality, affordable healthcare solutions while maintaining strict compliance with regulatory and quality standards.
Operating from its Savli (Vadodara) facility, Stellar Formulations follows robust GMP systems, quality assurance frameworks, and modern manufacturing practices. The organization emphasizes operational excellence, regulatory compliance, and continuous improvement across all functions.
Working at Stellar Formulations provides exposure to structured pharmaceutical operations, regulated manufacturing environments, and opportunities to contribute directly to safe and effective healthcare products.
Job Role & Responsibilities
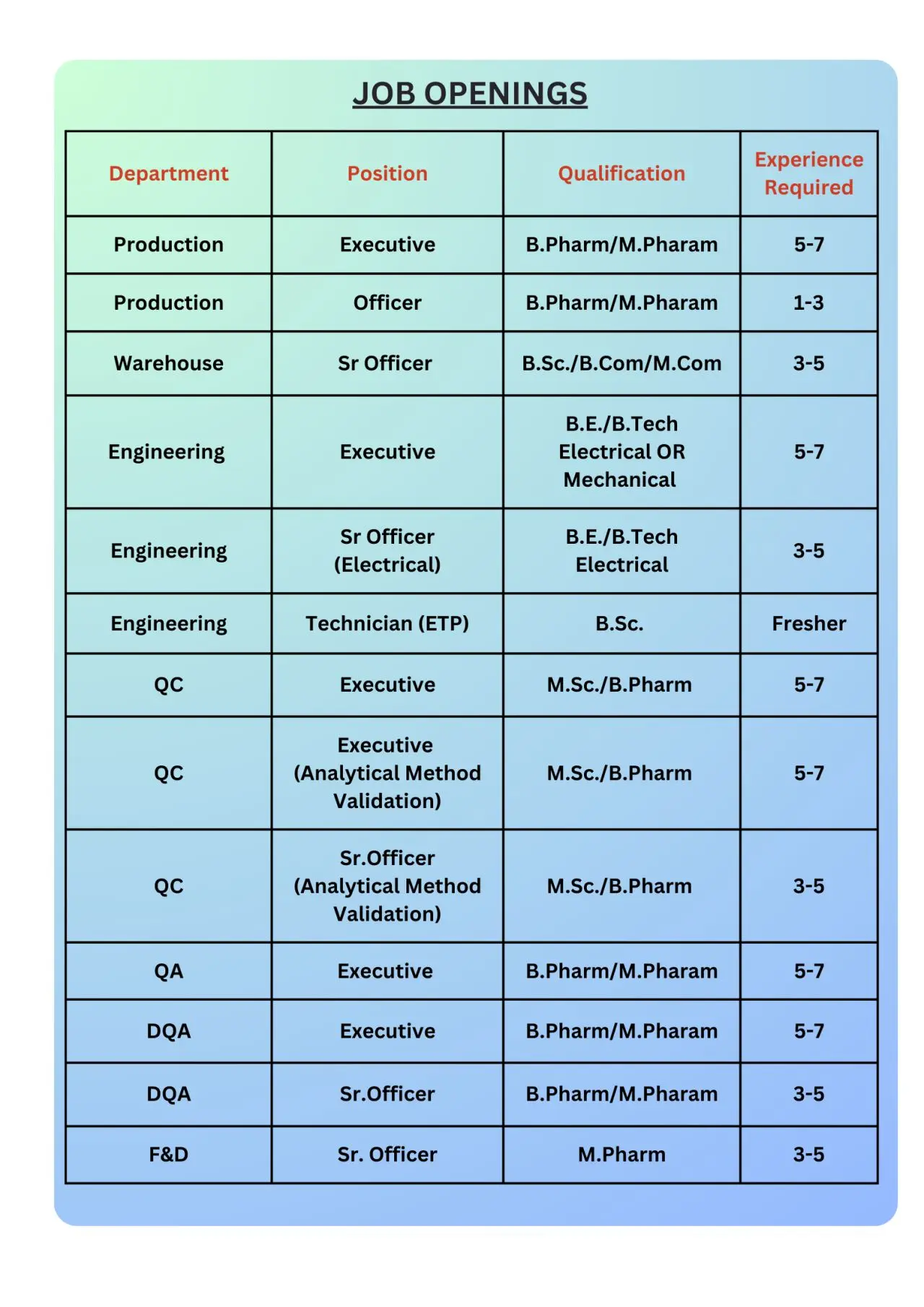
Stellar Formulations is hiring for the following departments and positions:
Production
Executive (5–7 Years) | Officer (1–3 Years)
- Execute and supervise OSD manufacturing activities.
- Operate granulation, compression, coating, and blending equipment.
- Ensure compliance with GMP and production SOPs.
- Maintain batch manufacturing records and documentation.
Warehouse
Senior Officer (3–5 Years)
- Manage raw material, packing material, and finished goods inventory.
- Ensure compliance with GMP storage and material handling practices.
- Coordinate material movement and documentation.
Engineering
Executive (5–7 Years)
Senior Officer – Electrical (3–5 Years)
Technician – ETP (Fresher)
- Maintain and troubleshoot manufacturing and utility equipment.
- Handle preventive and breakdown maintenance.
- Support electrical systems, utilities, and ETP operations.
- Ensure safety, compliance, and equipment reliability.
Quality Control (QC)
Executive (5–7 Years)
Executive – Analytical Method Validation (5–7 Years)
Senior Officer – Analytical Method Validation (3–5 Years)
- Perform routine and non-routine analysis of raw materials and finished products.
- Operate analytical instruments such as HPLC, GC, UV.
- Execute analytical method validation activities.
- Maintain laboratory documentation and data integrity.
Quality Assurance (QA)
Executive (5–7 Years)
- Oversee QA activities across manufacturing and quality systems.
- Review and approve BMRs, BPRs, and SOPs.
- Handle deviations, change controls, and CAPA.
Documentation & Other Areas
DQA – Executive (5–7 Years)
DOA – Senior Officer (3–5 Years)
- Manage documentation quality assurance and document control.
- Support audit readiness and compliance activities.
Formulation & Development (F&D)
Senior Officer (3–5 Years)
- Support formulation development and scale-up activities for OSD products.
- Prepare development reports and technical documentation.
Eligibility / Qualifications
Required Education
Candidates must possess one of the following qualifications based on the role:
B.Pharm, M.Pharm, M.Sc, B.Sc, B.Com, M.Com, B.E, B.Tech (Mechanical or Electrical)
Relevant Courses (comma-separated):
Pharmacy, Pharmaceutics, Pharmaceutical Sciences, Chemistry, Analytical Chemistry, Mechanical Engineering, Electrical Engineering, Commerce
Experience Requirement
- Fresher to 7 years, depending on role
- Mandatory exposure to OSD formulation for all applicable positions
Location & Salary
Job Location: Savli, Vadodara, Gujarat
Salary: Competitive and aligned with industry standards based on role, experience, and technical expertise.
Interview Details
Interview Dates: 10th January 2026 (Saturday) & 11th January 2026 (Sunday)
Time: 09:30 AM to 05:00 PM
Interview Venue:
Stellar Formulations Industries Pvt Ltd
Plot No. 83A, Alindra GIDC Estate
Taluka Savli, District Vadodara, Gujarat
Application Process
Interested candidates can attend the walk-in interview with the following documents:
- Updated resume
- Current salary proof
- Passport-size photographs
Candidates unable to attend the walk-in may share their resume via:
- Email: hr.savli_u1@stellarformulations.in
- Contact: +91 6357130094 / 6357313009
Why This Opportunity Matters
OSD formulation manufacturing remains a core segment of the pharmaceutical industry. Roles across Production, QA, QC, Engineering, and F&D directly impact product quality, regulatory compliance, and patient safety.
Joining Stellar Formulations allows professionals to strengthen their expertise in solid oral dosage manufacturing while contributing to reliable and compliant healthcare product supply.
Frequently Asked Questions (FAQs)
Is OSD formulation experience mandatory?
Yes. Exposure to solid oral dosage formulation is essential for all applicable roles.
Can freshers apply?
Freshers are eligible for the ETP Technician role.
What qualifications are accepted?
B.Pharm, M.Pharm, M.Sc, B.Sc, B.E, B.Tech, and commerce degrees depending on the role.
Is this a direct company hiring?
Yes. This is a direct walk-in interview conducted by Stellar Formulations Industries.
Summary Table
| Company | Stellar Formulations Industries Pvt Ltd |
|---|---|
| Vacancies | Production, QC, QA, Engineering, Warehouse, F&D |
| Required Education | B.Pharm, M.Pharm, M.Sc, B.E/B.Tech, B.Sc, B.Com |
| Experience | Fresher to 7 years (OSD experience mandatory) |