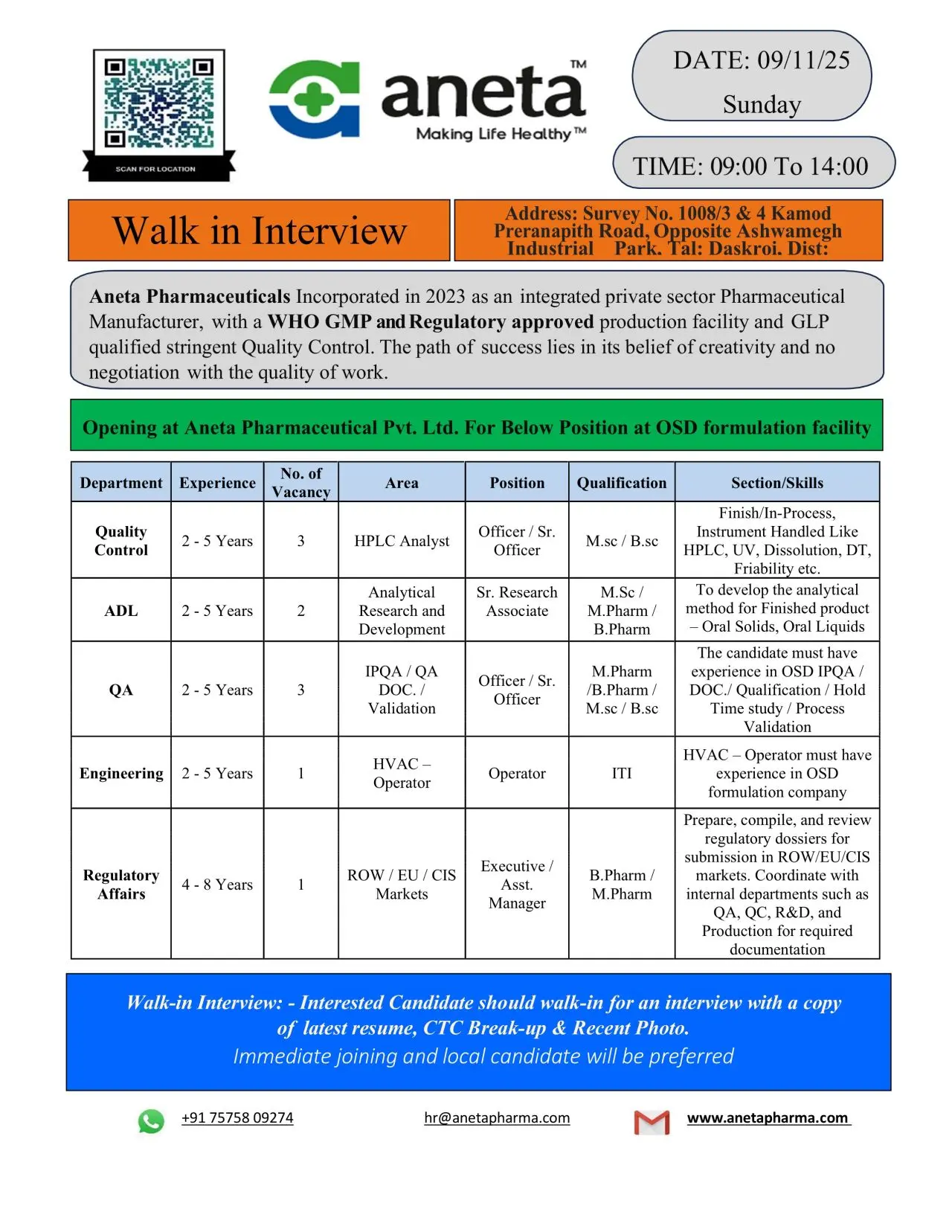
Aneta Walk-in QA, QC, AR&D, Engineering, and Regulatory Affairs

- B.Pharm, M.Pharm Openings – Aneta Pharmaceuticals OSD Facility, Ahmedabad
- Company Overview
- Walk-in Interview Details
- Job Role & Responsibilities
- 1. HPLC Analyst – Quality Control (QC)
- 2. Analytical Research & Development (AR&D)
- 3. Quality Assurance (QA)
- 4. Engineering (HVAC Operator)
- 5. Regulatory Affairs
- Eligibility / Qualifications
- Location & Salary
- Application Process
- Why Join Aneta Pharmaceuticals
- FAQs
B.Pharm, M.Pharm Openings – Aneta Pharmaceuticals OSD Facility, Ahmedabad
Walk-in Interview at Aneta Pharmaceuticals, Ahmedabad for QA, QC, AR&D, Engineering, and Regulatory Affairs roles. Apply with 2–8 years experience.
Aneta Pharmaceuticals Pvt. Ltd., an integrated WHO-GMP and GLP-approved pharmaceutical manufacturer, invites talented professionals to attend a Walk-in Interview for multiple positions across Quality Assurance (QA), Quality Control (QC), Analytical Research & Development (AR&D), Engineering, and Regulatory Affairs at its OSD Formulation Facility in Ahmedabad, Gujarat.
If you are passionate about quality, innovation, and pharmaceutical excellence, this is your opportunity to join one of India’s emerging leaders in formulation development and manufacturing.
Company Overview
Aneta Pharmaceuticals Pvt. Ltd., incorporated in 2023, has quickly established itself as a trusted pharmaceutical manufacturer committed to Making Life Healthy. The company’s integrated production facility is compliant with WHO-GMP, equipped with modern analytical instruments, and qualified under Good Laboratory Practices (GLP).
Aneta focuses on Oral Solid Dosage (OSD) and Oral Liquid formulations, serving both domestic and regulated international markets. Its core strength lies in innovation, precision, and an uncompromising commitment to quality. The organization promotes a growth-oriented culture where every professional contributes to operational excellence and scientific advancement.
Walk-in Interview Details
Date: 9th November 2025 (Sunday)
Time: 09:00 AM to 02:00 PM
Venue: Survey No. 1008/3 & 4, Kamod Preranapith Road, Opposite Ashwamegh Industrial Park, Tal: Daskroi, Dist: Ahmedabad, Gujarat
Contact: +91 75758 09274
Email: hr@anetapharma.com
Job Role & Responsibilities
1. HPLC Analyst – Quality Control (QC)
Position: Officer / Sr. Officer
Qualification: M.Sc / B.Sc (Chemistry)
Experience: 2–5 Years
Vacancies: 2
Key Responsibilities:
- Perform instrumental analysis using HPLC, UV, Dissolution, DT, and Friability equipment.
- Conduct finish product and in-process testing for OSD formulations.
- Ensure compliance with GLP and cGMP standards.
- Maintain analytical documentation, calibration logs, and result records.
Preferred Skills:
- Strong hands-on experience with analytical instruments.
- Knowledge of OOS/OOT investigations and laboratory safety procedures.
2. Analytical Research & Development (AR&D)
Position: Sr. Research Associate
Qualification: M.Sc / M.Pharm / B.Pharm
Experience: 2–5 Years
Vacancies: 3
Key Responsibilities:
- Develop and validate analytical methods for finished products (Oral Solids, Oral Liquids).
- Prepare method validation protocols, reports, and analytical summaries.
- Support formulation and stability studies.
- Collaborate with QA/QC teams to ensure method transfer and compliance.
Preferred Skills:
- Experience in analytical method development and validation.
- Familiarity with ICH Q2(R1) guidelines and analytical documentation.
3. Quality Assurance (QA)
Position: Officer / Sr. Officer (IPQA / QA Documentation / Validation)
Qualification: M.Pharm / B.Pharm / M.Sc
Experience: 2–5 Years
Vacancies: 3
Key Responsibilities:
- Oversee IPQA activities for OSD manufacturing.
- Handle process validation, qualification, and hold-time studies.
- Prepare and review SOPs, BMRs, and validation reports.
- Maintain compliance with regulatory and cGMP standards.
Preferred Skills:
- Experience in QA documentation and audit preparedness.
- Good understanding of data integrity principles and GxP requirements.
4. Engineering (HVAC Operator)
Position: Operator
Qualification: ITI / Diploma (Mechanical / Electrical)
Experience: 2–5 Years
Vacancies: 1
Key Responsibilities:
- Operate and maintain HVAC systems in OSD formulation units.
- Monitor differential pressures, humidity, and temperature control systems.
- Support preventive maintenance and calibration schedules.
Preferred Skills:
- Prior experience in OSD formulation company.
- Understanding of cleanroom requirements and regulatory compliance.
5. Regulatory Affairs
Position: Executive / Assistant Manager
Qualification: B.Pharm / M.Pharm
Experience: 4–8 Years
Vacancies: 1
Key Responsibilities:
- Prepare, compile, and review regulatory dossiers for ROW, EU, and CIS markets.
- Coordinate with QA, QC, R&D, and Production for required documentation.
- Support regulatory submissions and query responses.
- Stay updated with evolving regulatory guidelines and submission formats.
Preferred Skills:
- Strong knowledge of CTD/eCTD documentation and global regulatory pathways.
- Familiarity with ROW/EU/CIS market regulations.
Eligibility / Qualifications
Educational Requirements:
M.Pharm, B.Pharm, M.Sc (Chemistry), B.Sc (Chemistry), ITI/Diploma (Mechanical/Electrical)
Experience:
2–8 years in OSD formulation, analytical, QA, or regulatory functions.
Location & Salary
Work Location: Ahmedabad, Gujarat
Employment Type: Full-time
Facility Type: OSD Formulation Manufacturing Facility
Compensation: Competitive salary with benefits and growth opportunities.
Application Process
Walk-in Interview:
Interested candidates should attend the walk-in drive with a copy of their latest resume, recent photograph, and CTC breakup. Immediate joiners and local candidates will be preferred.
Date: 9th November 2025 (Sunday)
Time: 09:00 AM – 02:00 PM
Venue: Survey No. 1008/3 & 4, Kamod Preranapith Road, Opposite Ashwamegh Industrial Park, Daskroi, Ahmedabad, Gujarat
Contact: +91 75758 09274
Email: hr@anetapharma.com
Why Join Aneta Pharmaceuticals
- Work with a WHO-GMP and GLP-approved organization committed to high-quality standards.
- Exposure to regulatory and R&D operations within a rapidly growing pharma manufacturer.
- Opportunities for professional growth and cross-functional learning.
- Competitive pay, excellent work-life balance, and a culture built on innovation and compliance.
FAQs
Q1. What positions are open at Aneta Pharmaceuticals?
A1. Openings are available in QA, QC, AR&D, Engineering, and Regulatory Affairs departments.
Q2. What qualifications are required?
A2. B.Pharm, M.Pharm, M.Sc, B.Sc, ITI, or Diploma in relevant disciplines.
Q3. Is OSD experience mandatory?
A3. Yes, candidates with experience in OSD formulation manufacturing will be given preference.
Q4. Where is the interview being held?
A4. The interview will be conducted at Aneta Pharmaceuticals, Kamod Preranapith Road, Ahmedabad.
Q5. Can freshers apply?
A5. No, this drive is open to candidates with 2–8 years of relevant experience.
Q6. Is immediate joining preferred?
A6. Yes, immediate joiners and local candidates will be given priority.
| Company | Aneta Pharmaceuticals Pvt. Ltd. |
|---|---|
| Vacancies | QA, QC, AR&D, HVAC Operator, Regulatory Affairs |
| Required Education | B.Pharm, M.Pharm, M.Sc, B.Sc, ITI/Diploma |
| Experience | 2–8 Years |
| Location | Ahmedabad, Gujarat |