Helios Hiring QA, QC, and Production officers

- B.Pharm QA, QC, Production Openings | Helios Pharmaceuticals | Baddi
- Company Overview
- Job Role & Responsibilities
- Production Department
- Quality Assurance (QA) Department
- Quality Control (QC) Department
- Eligibility / Qualifications
- Location & Salary
- Application Process
- Why Join Helios Pharmaceuticals?
- FAQs
- Job Summary Table
B.Pharm QA, QC, Production Openings | Helios Pharmaceuticals | Baddi
Helios Pharmaceuticals hiring QA, QC, and Production officers at Baddi, HP. B.Pharm/B.Sc graduates with 2–8 yrs exp.
Helios Pharmaceuticals, a rapidly growing pharmaceutical manufacturing organization, is inviting skilled professionals to join its Production, Quality Assurance (QA), and Quality Control (QC) teams at its state-of-the-art facility in Baddi, Himachal Pradesh. These roles offer a great opportunity for pharma professionals to advance their careers in a regulated, growth-oriented environment.
Company Overview
Helios Pharmaceuticals Pvt. Ltd. is known for its robust portfolio of formulations, consistent adherence to GMP, WHO, and regulatory standards, and dedication to innovation in drug manufacturing. Located in the industrial hub of Baddi, Solan (Himachal Pradesh), Helios specializes in high-quality formulations catering to domestic and international markets.
The company’s commitment to operational excellence, continuous improvement, and regulatory compliance has positioned it as a trusted player in the Indian pharmaceutical manufacturing landscape.
Job Role & Responsibilities
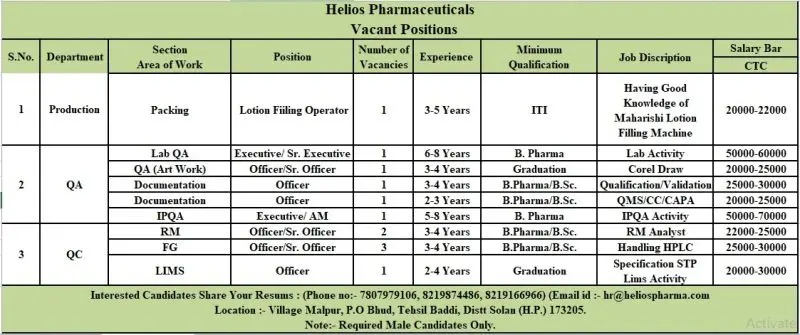
Production Department
Position: Lotion Filling Operator
Vacancy: 1
Experience: 3–5 Years
Qualification: ITI / Diploma / Graduate
Key Responsibilities:
- Operate and maintain Maharishi Lotion Filling Machines efficiently.
- Monitor filling operations for accuracy, safety, and GMP compliance.
- Support preventive maintenance and calibration of equipment.
- Ensure all batch and process documentation is completed as per SOP.
Quality Assurance (QA) Department
Open Positions:
- Executive / Sr. Executive (Art Work – Lab QA) — 1 Vacancy
Experience: 6–8 Years | Qualification: B.Pharm / Graduate
Key Role: Manage artwork and documentation processes in QA using CorelDRAW; ensure compliance with regulatory design and labeling guidelines. - Officer (Documentation) — 1 Vacancy
Experience: 3–4 Years | Qualification: B.Pharm / B.Sc
Key Role: Handle QA documentation, validation reports, SOP updates, and audit preparation activities. - Officer / Sr. Officer (Qualification / Validation) — 1 Vacancy
Experience: 2–3 Years | Qualification: B.Pharm / B.Sc
Key Role: Execute equipment and process qualification, maintain validation records, and support audits. - Executive / Assistant Manager (QMS / CC / CAPA) — 1 Vacancy
Experience: 2–3 Years | Qualification: B.Pharm / B.Sc
Key Role: Manage Change Control, CAPA, and QMS documentation systems ensuring timely closure of deviations. - Officer / Sr. Officer (IPQA) — 2 Vacancies
Experience: 5–8 Years | Qualification: B.Pharm
Key Role: Oversee in-process QA activities, batch review, line clearance, and compliance checks in manufacturing and packing areas.
Quality Control (QC) Department
Open Positions:
- Officer / Sr. Officer (Raw Material) — 3 Vacancies
Experience: 3–4 Years | Qualification: B.Pharm / B.Sc
Key Role: Perform RM sampling, analysis, and documentation as per specifications. - Officer / Sr. Officer (Finished Goods) — 1 Vacancy
Experience: 3–4 Years | Qualification: B.Pharm / B.Sc
Key Role: Conduct finished goods testing, handle HPLC operations, and review analytical data. - Officer (LIMS) — 1 Vacancy
Experience: 2–4 Years | Qualification: Graduate
Key Role: Manage LIMS data, generate specifications/STPs, and support QC documentation.
Eligibility / Qualifications
Education:
- B.Pharm / M.Pharm / B.Sc / Graduate (depending on department)
Experience:
- 2–8 years of relevant experience in pharma manufacturing, QA, or QC operations.
Courses (comma-separated): Pharmaceutical Analysis, Industrial Pharmacy, Quality Management Systems, GMP Compliance, HPLC Operation, Documentation Practices, Process Validation.
Key Competencies:
- Strong understanding of GMP / GLP standards.
- Hands-on experience in analytical instruments like HPLC, UV, FTIR.
- Proficiency in documentation and audit readiness.
- Excellent teamwork, communication, and problem-solving skills.
Location & Salary
Job Location: Village Malpur, P.O. Bhud, Tehsil Baddi, Dist. Solan (H.P.) – 173205
Salary Range: ₹22,000 – ₹30,000 per month (depending on role and experience)
Employment Type: Full-time
Gender Requirement: Male candidates only
Application Process
Interested candidates may send their updated resumes to:
Email: hr@heliospharma.com
Contact: 7807979106 / 8219874486 / 8219166966
Please mention the department and position title in the subject line (e.g., “Application – QA Officer / Production”).
Why Join Helios Pharmaceuticals?
- Exposure to GMP, WHO, and regulatory audits.
- Opportunity to work with advanced analytical systems and manufacturing equipment.
- Collaborative work culture focused on quality and compliance.
- Growth-oriented environment with cross-functional learning.
FAQs
1. What is the location of Helios Pharmaceuticals?
The company is located in Village Malpur, P.O. Bhud, Tehsil Baddi, Solan District, Himachal Pradesh.
2. Which roles are currently open?
Openings include Production Operator, QA Officers, Validation Executives, IPQA Officers, and QC Analysts.
3. What is the experience requirement?
Candidates with 2–8 years of relevant pharma experience are eligible.
4. What is the pay range?
Salary ranges from ₹22,000 to ₹30,000 per month, based on position and experience.
5. How do I apply?
Send your CV to hr@heliospharma.com or contact the HR numbers listed above.
Job Summary Table
| Category | Details |
|---|---|
| Company | Helios Pharmaceuticals Pvt. Ltd. |
| Vacancies | Multiple – QA, QC, Production |
| Required Education | B.Pharm / M.Pharm / B.Sc / Graduate |
| Experience | 2–8 Years |
| Location | Baddi, Himachal Pradesh |
| hr@heliospharma.com | |
| Contact | 7807979106 / 8219874486 / 8219166966 |
To apply for this job email your details to hr@heliospharma.com


