Anthea Pharma Walk-in QC Executive & Trainee

- Anthea Pharma QC Executive & Trainee Openings – Hyderabad
- Company Overview
- Job Role & Responsibilities
- Position: Executive – Quality Control
- Position: Trainee – Quality Control
- Eligibility / Qualifications
- Location & Salary
- Application Process
- Key Highlights
- FAQs
Anthea Pharma QC Executive & Trainee Openings – Hyderabad
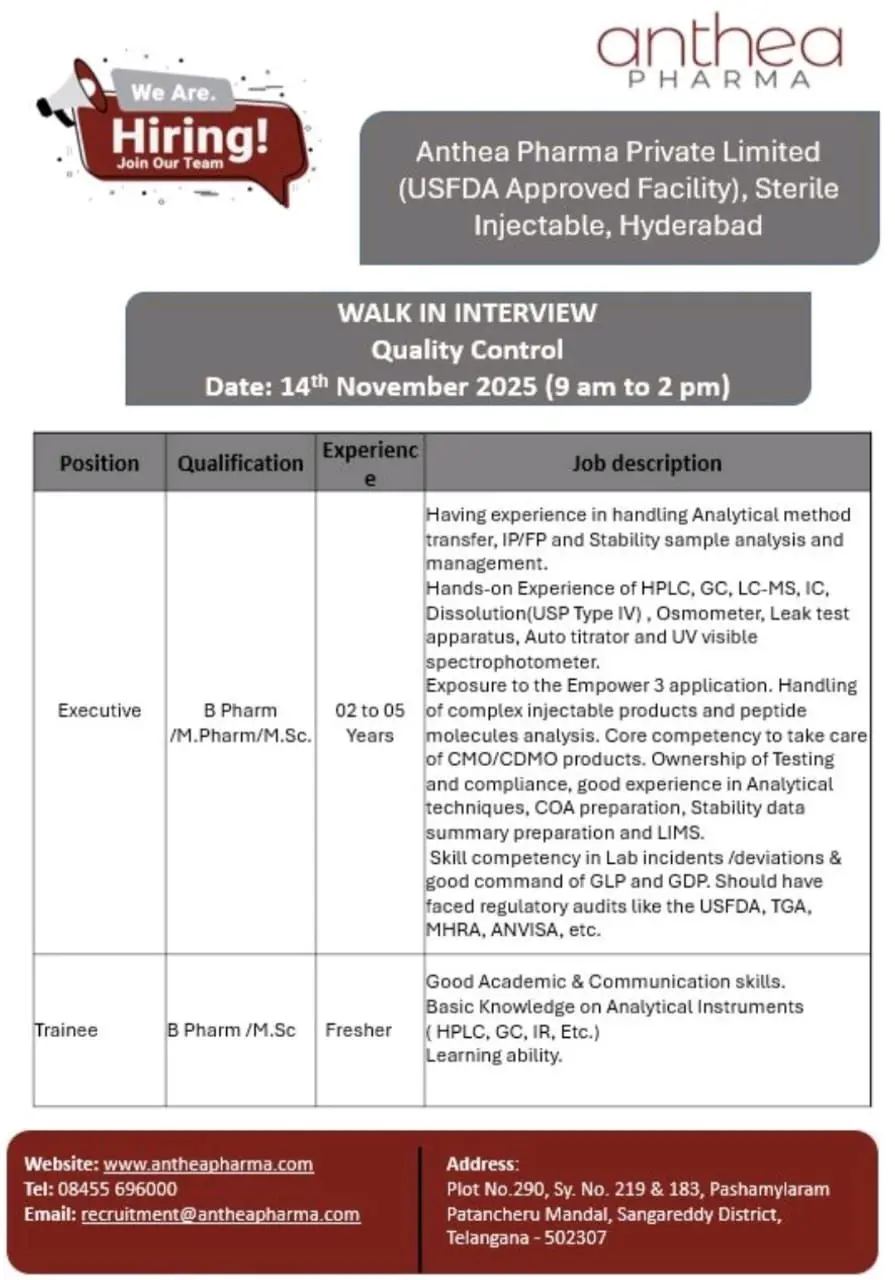
B.Pharm/M.Pharm/M.Sc openings in Quality Control – Walk-in on 14-Nov-2025, Hyderabad (USFDA approved sterile injectable facility).
Anthea Pharma Pvt. Ltd., a USFDA-approved sterile injectable manufacturing company based in Hyderabad, is conducting a walk-in drive for Quality Control (QC) professionals and trainees on 14th November 2025. This opportunity is ideal for candidates with analytical chemistry skills seeking to work in a regulated environment with advanced instruments and global exposure.
Company Overview
Anthea Pharma Private Limited is a fast-growing sterile injectable manufacturer recognized for its high-quality manufacturing standards and global regulatory compliance. With an USFDA, TGA, MHRA, and ANVISA-approved facility, Anthea Pharma specializes in complex injectables and peptide molecule formulations. The company is committed to innovation, regulatory excellence, and continuous improvement in sterile manufacturing.
Working here means joining a team driven by precision, data integrity, and advanced analytical science—core pillars of regulatory compliance and patient safety in the pharmaceutical industry.
Job Role & Responsibilities
Position: Executive – Quality Control
Experience: 2–5 years
Qualification: B.Pharm / M.Pharm / M.Sc (Analytical Chemistry, Organic Chemistry, Pharmaceutical Analysis)
Core Responsibilities:
- Perform analytical method transfer, in-process (IP), finished product (FP), and stability sample analysis.
- Handle advanced instruments including HPLC, GC, LC-MS, IC, Dissolution (USP Type IV), Osmometer, Leak Test Apparatus, Auto Titrator, and UV-Visible Spectrophotometer.
- Manage Empower 3 software for analytical data processing.
- Conduct testing and data review for CMO/CDMO projects.
- Prepare COA, stability data summaries, and ensure LIMS data integrity.
- Participate in laboratory incident handling, deviations, and CAPA documentation.
- Maintain compliance with GLP and GDP requirements.
- Ensure readiness for regulatory audits (USFDA, MHRA, TGA, ANVISA, WHO, etc.).
Preferred Skills:
- Strong command of analytical techniques and method validation.
- Experience with regulatory documentation and audit preparedness.
- Clear communication and strong academic foundation in pharmaceutical sciences.
Position: Trainee – Quality Control
Experience: Freshers
Qualification: B.Pharm / M.Sc
Responsibilities:
- Learn and assist in analytical instrument operation (HPLC, GC, IR, UV).
- Support analytical method execution under supervision.
- Maintain lab notebooks, calibration records, and instrument logbooks.
- Participate in training on GMP documentation and lab safety practices.
Skills Required:
- Basic understanding of analytical chemistry.
- Curiosity to learn new analytical techniques.
- Good communication and teamwork skills.
Eligibility / Qualifications
Required Education: B.Pharm, M.Pharm, M.Sc (Analytical Chemistry / Pharmaceutical Analysis / Organic Chemistry)
Relevant Courses (comma-separated):
B.Pharm, M.Pharm, M.Sc Analytical Chemistry, M.Sc Organic Chemistry, M.Sc Pharmaceutical Analysis, PG Diploma in Quality Control, PG Diploma in Pharmaceutical Analysis, B.Sc Chemistry, M.Sc Biochemistry
Experience:
- Executive: 2–5 years in QC analytical labs (preferably injectables / formulations)
- Trainee: Freshers with basic instrument knowledge
Location & Salary
Venue:
Anthea Pharma Pvt. Ltd.
Plot No. 290, Sy. No. 219 & 183, Pashamylaram, Patancheru Mandal,
Sangareddy District, Telangana – 502307.
Walk-in Date & Time: 14th November 2025 | 9:00 AM – 2:00 PM.
Salary: Based on qualification and experience, aligned with industry standards for QC roles in sterile manufacturing. Typically, Executive roles earn between INR 3.6–6 LPA; Trainee packages vary per qualification.
Application Process
- Attend the walk-in interview directly at the Anthea Pharma facility on 14-Nov-2025 between 9 AM and 2 PM.
- Bring the following documents:
- Updated CV
- Aadhaar & PAN Card
- Educational certificates (original & photocopy)
- One passport-size photo
- You can also email your updated resume to recruitment@antheapharma.com for consideration.
- For queries, contact: +91 8455 696000.
Key Highlights
- Opportunity to work in a USFDA-approved sterile injectable facility.
- Exposure to advanced analytical instrumentation and data systems like Empower 3 and LIMS.
- Hands-on learning in QC operations under GLP, GDP, and global regulatory standards.
- Fast career growth in analytical sciences, QA/QC, and regulatory functions.
- Direct walk-in with immediate interview opportunity.
FAQs
Q: Who can attend the walk-in?
A: Candidates with B.Pharm, M.Pharm, or M.Sc degrees and relevant analytical or QC experience, as well as freshers for trainee positions.
Q: What instruments should I be familiar with?
A: HPLC, GC, LC-MS, UV, Dissolution (USP Type IV), Osmometer, Auto Titrator, IC, and Leak Test Apparatus.
Q: What regulatory exposure is required?
A: Candidates should have knowledge of or experience in handling audits like USFDA, MHRA, TGA, and ANVISA.
Q: Can freshers apply?
A: Yes, B.Pharm and M.Sc freshers can apply for QC Trainee positions.
Q: Is prior Empower 3 experience mandatory?
A: It’s preferred for Executive roles but not mandatory for trainees.
Q: What is the company culture like?
A: Anthea Pharma promotes learning, regulatory discipline, and scientific excellence in a collaborative work environment.
Vertical Summary Table
+------------------------------+----------------------------------------------------------------+
| Company | Anthea Pharma Private Limited |
+------------------------------+----------------------------------------------------------------+
| Vacancies | Executive (QC), Trainee (QC) |
+------------------------------+----------------------------------------------------------------+
| Required Education | B.Pharm, M.Pharm, M.Sc (Analytical/Pharmaceutical Chemistry) |
+------------------------------+----------------------------------------------------------------+
| Experience | 0–5 years (Injectables/Analytical QC preferred) |
+------------------------------+----------------------------------------------------------------+