Hopecure Lifecare Hiring QA/QC Officer
- Company Overview
- Job Role & Responsibilities
- QA — Officer / Executive (Documentation, BMR review)
- QC — Shift In‑charge (Second/Rotational Shift)
- Eligibility / Qualifications
- Location & Salary
- Application Process
- How to Prepare a Strong Application
- Why this role matters (EEAT Alignment)
- High‑Value Keywords Included Naturally
- FAQs
M.Sc QA/QC Officer — Hopecure Lifecare Ahmedabad
M.Sc graduates: 2 QA/QC openings at Hopecure Lifecare, Ahmedabad. Freshers/1 year experience. Apply via email.
Hopecure Lifecare Devices in Ahmedabad is hiring QA and QC professionals to support its growing medical devices operations. These are entry-level to early-career roles ideal for M.Sc graduates in Microbiology, Biotechnology, Chemistry, or related science streams. If you want hands-on exposure to QA documentation, IPQC, validation support, and line clearance for medical device manufacturing, this is an immediate opportunity.
Company Overview
Hopecure Lifecare Devices is a medical device manufacturer focused on quality, compliance, and patient safety. Located near Concord Biotech Unit-II, Valthera, Ahmedabad, the company develops, manufactures, and supplies devices that require strict adherence to quality systems and regulatory standards. Working at Hopecure gives you exposure to device-specific QA/QC practices, document control, validation activities, and clean production environments — experience that is directly transferable to regulated manufacturing and device regulatory submissions.
Job Role & Responsibilities
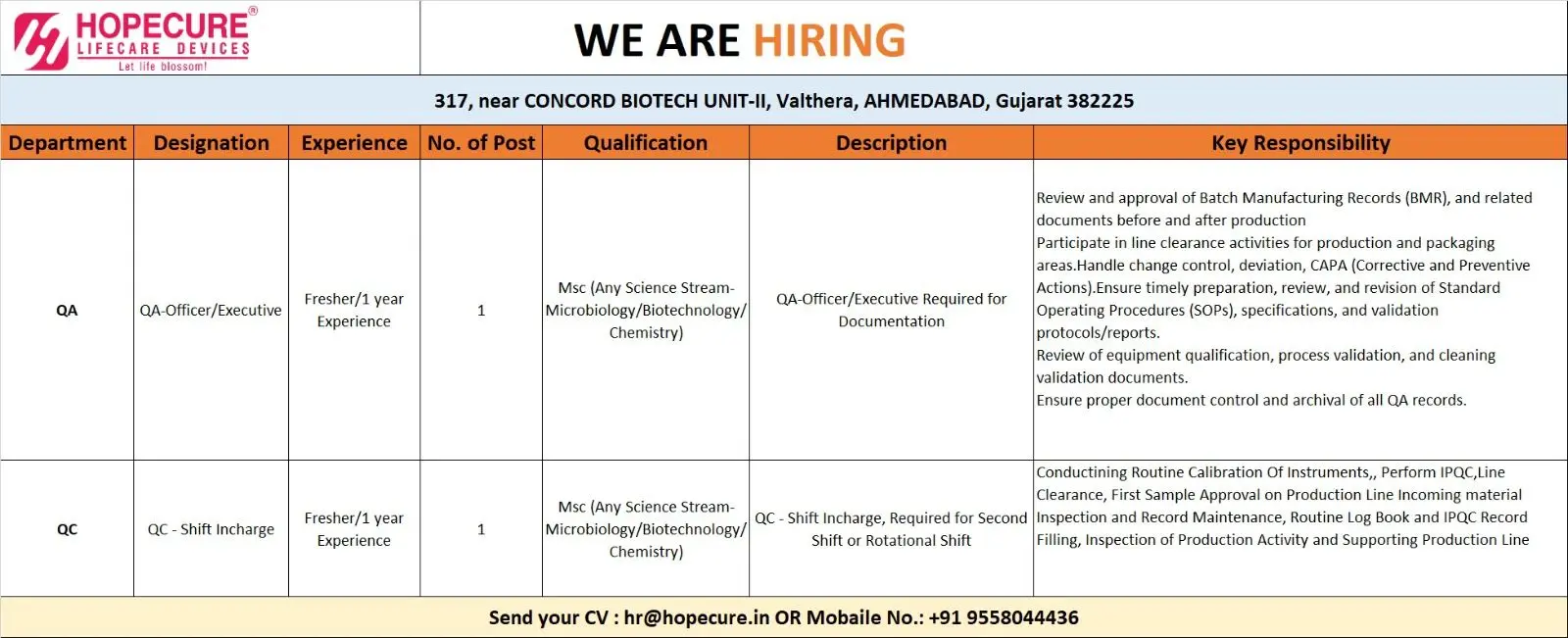
QA — Officer / Executive (Documentation, BMR review)
No. of posts: 1
Experience: Fresher / 1 year
Qualification: M.Sc (Microbiology / Biotechnology / Chemistry / any Science stream)
Key responsibilities:
- Review and approve Batch Manufacturing Records (BMR) before and after production.
- Participate in line clearance activities for production and packaging areas.
- Manage change controls, deviations, and CAPA (Corrective and Preventive Actions).
- Prepare, review, and revise Standard Operating Procedures (SOPs), specifications, and validation protocols/reports.
- Support equipment qualification, process validation, and cleaning validation documentation.
- Maintain document control, archival of QA records, and ensure ALCOA+ data integrity for QA files.
- Coordinate with production, QC, and engineering teams during investigations and audits.
This role requires methodical documentation habits, familiarity with GMP/GDP principles, and the ability to escalate and close quality issues with corrective actions.
QC — Shift In‑charge (Second/Rotational Shift)
No. of posts: 1
Experience: Fresher / 1 year
Qualification: M.Sc (Microbiology / Biotechnology / Chemistry / any Science stream)
Key responsibilities:
- Lead QC activities during the assigned shift: incoming material inspection, IPQC (In-Process Quality Control), and first sample approvals on the production line.
- Conduct routine calibration checks of instruments used in QC and production support.
- Maintain routine logbooks, IPQC records, and inspection documentation.
- Inspect production activities, support production lines, and ensure compliance with SOPs and aseptic/clean area behavior where applicable.
- Coordinate with QA for deviations, performed sampling, and completion of QC release paperwork.
The shift-in-charge role emphasizes operational ownership, quick decision-making, and solid record-keeping under production timelines.
Eligibility / Qualifications
- Required qualification: M.Sc in Microbiology, Biotechnology, Chemistry, or related science streams.
- Experience: Freshers and candidates with up to 1 year of relevant experience are welcome.
- Technical skills: Basic understanding of GMP principles, familiarity with BMR/BPR, IPQC practices, instrument calibration, and documentation control.
- Soft skills: Attention to detail, strong communication, teamwork, and readiness to work rotational or second shifts for QC role.
Relevant courses (comma-separated): M.Sc Microbiology, M.Sc Biotechnology, M.Sc Chemistry, B.Sc Life Sciences, Postgraduate Diploma in QA/QC, Diploma in Microbial Technology, B.Pharm (if applicable)
Location & Salary
- Location: 317, near Concord Biotech Unit-II, Valthera, Ahmedabad, Gujarat 382225.
- Work model: On-site roles; QC in shift pattern (second or rotational shift for QC Shift In‑charge).
- Salary: Not specified in the HR brief. Compensation will be as per company policies and candidate experience.
Application Process
Send your updated CV to: hr@hopecure.in
Or contact via mobile: +91 95580 44436
Email subject suggestion: Application — QA Officer / QC Shift In‑charge — [Your Name]
Include a short cover sentence stating your availability (immediate or notice period), preferred role (QA or QC), and any prior exposure to device manufacturing or GMP environments.
How to Prepare a Strong Application
- Highlight any lab internships, industrial projects, or academic projects related to manufacturing, validation, or quality systems.
- For QA applicants: list any experience with BMR/BPR, SOP drafting, CAPA handling, or audit support activities.
- For QC applicants: emphasise hands-on exposure to IPQC, sampling, logbook maintenance, and instrument handling/calibration.
- Keep the CV concise (1–2 pages), use clear section headings, and provide contactable references if available.
Why this role matters (EEAT Alignment)
Quality Assurance and Quality Control are the backbone of medical device manufacturing. Accurate document control, IPQC checks, and validated processes ensure product safety and regulatory compliance. At Hopecure Lifecare Devices, these roles help maintain manufacturing integrity, support regulatory readiness, and protect patient outcomes — aligning with Google’s EEAT principles by demonstrating expertise, authoritativeness, and trustworthiness in device quality systems.
High‑Value Keywords Included Naturally
medical device QA jobs, quality assurance officer, QC shift incharge, IPQC jobs, GMP validation, SOP documentation, CAPA management, device manufacturing careers, QC laboratory jobs, BMR review jobs.
FAQs
Q: Are freshers eligible to apply?
A: Yes. Both QA Officer and QC Shift In‑charge roles accept freshers or candidates with up to 1 year of experience.
Q: Is the QC role a night shift?
A: The QC Shift In‑charge is required for second shift or rotational shift; specific shift timings will be communicated by HR.
Q: What documents should I attach?
A: Updated CV, academic transcripts (if available), and any experience or internship certificates.
Q: Is prior medical device experience mandatory?
A: Not mandatory for freshers, but device or GMP exposure will be an advantage.
Q: How soon can I expect a response?
A: HR will review CVs and contact shortlisted candidates for next steps. Include your availability and notice period to speed up processing.
| Category | Details |
|---|---|
| Company | Hopecure Lifecare Devices |
| Vacancies | QA Officer/Executive (Documentation) — 1, QC Shift In‑charge — 1 |
| Required Education | M.Sc Microbiology, M.Sc Biotechnology, M.Sc Chemistry, B.Sc Life Sciences, PG Diploma QA/QC |
| Experience | Fresher to 1 year |
To apply for this job email your details to hr@hopecure.in


