Damaira walk-in Production, RA, QA, QC, Micro, BD, Purchase, Apprenticeship

- Company Overview
- Job Role & Responsibilities
- Production – Assistant
- Production – Manufacturing Executive
- Regulatory Affairs – Dossier Preparation
- Business Development
- Microbiology
- Quality Control (QC)
- Quality Assurance (QA)
- Purchase
- Graduate Apprenticeship Trainee
- Eligibility / Qualifications
- Accepted Courses
- Experience Requirements
- Location & Salary
- Application Process
- Walk-in Interview Details
- FAQs
- Are freshers eligible?
- What departments have openings?
- What is the experience range for QC and Microbiology?
- What qualifications are required for Production roles?
- Is MBA mandatory for Business Development?
- Summary Table
Pharma Openings for BSc BPharm ITI at Damaira Chandigarh
Damaira Pharmaceuticals hiring for Production, QA, QC, Regulatory, BD, Microbiology, and Apprenticeship roles at Chandigarh.
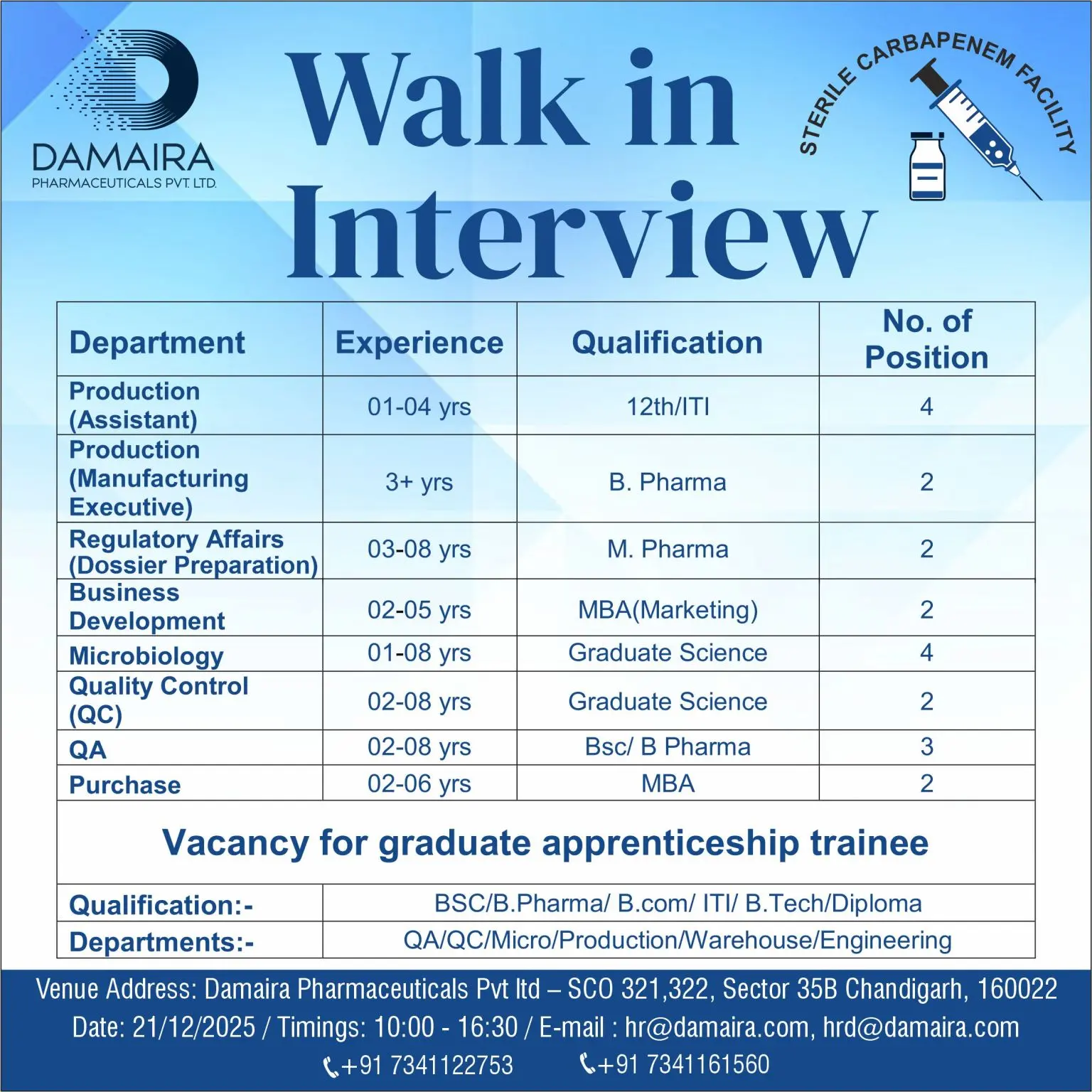
Damaira Pharmaceuticals Pvt. Ltd. is conducting a walk-in interview to fill multiple vacancies across Production, QA, QC, Regulatory Affairs, Microbiology, Business Development, Purchase, and Graduate Apprenticeship programs. The roles are suitable for candidates seeking long-term growth in sterile manufacturing, carbapenem production, regulatory documentation, laboratory testing, and operational support functions.
Company Overview
Damaira Pharmaceuticals operates specialized sterile and carbapenem manufacturing facilities supported by strong quality systems, modern infrastructure, and disciplined process controls. The company focuses on regulated market compliance, robust documentation practices, and a culture that encourages skill development across manufacturing, laboratory operations, and regulatory submissions.
Job Role & Responsibilities
Production – Assistant
Experience: 1–4 years
Qualification: 12th, ITI
Positions: 4
Responsibilities:
- Assist in sterile and carbapenem manufacturing operations.
- Handle basic production activities and support batch execution.
- Follow safety procedures and maintain plant hygiene.
Production – Manufacturing Executive
Experience: 3+ years
Qualification: BPharm
Positions: 2
Responsibilities:
- Execute manufacturing processes in sterile formulation areas.
- Ensure adherence to GMP, SOPs, and batch documentation.
- Coordinate with QA and Engineering for smooth operations.
Regulatory Affairs – Dossier Preparation
Experience: 3–8 years
Qualification: MPharm
Positions: 2
Responsibilities:
- Prepare regulatory dossiers as per international and domestic guidelines.
- Review product documentation, COAs, and technical files.
- Ensure regulatory compliance and timely submissions.
Business Development
Experience: 2–5 years
Qualification: MBA (Marketing)
Positions: 2
Responsibilities:
- Support market development and client outreach.
- Execute business strategies for product growth.
- Coordinate with internal teams for project execution.
Microbiology
Experience: 1–8 years
Qualification: Graduate in Science
Positions: 4
Responsibilities:
- Conduct microbiological testing for sterile products.
- Perform environmental monitoring and water testing.
- Maintain laboratory documentation and follow compliance standards.
Quality Control (QC)
Experience: 2–8 years
Qualification: Graduate in Science
Positions: 2
Responsibilities:
- Perform chemical and instrumental analysis for raw, in-process, and finished products.
- Support data documentation and ensure adherence to GMP.
Quality Assurance (QA)
Experience: 2–8 years
Qualification: BSc, BPharm
Positions: 3
Responsibilities:
- Handle documentation review, line clearance, change control, and deviation management.
- Maintain cGMP practices across the facility.
Purchase
Experience: 2–6 years
Qualification: MBA
Positions: 2
Responsibilities:
- Manage procurement, vendor coordination, and material sourcing.
- Maintain documentation and support cost optimization.
Graduate Apprenticeship Trainee
Qualification: BSc, BPharm, BCom, ITI, BTech, Diploma
Departments: QA, QC, Microbiology, Production, Warehouse, Engineering
Responsibilities:
- Support day-to-day departmental operations.
- Learn industry documentation, compliance, and basic operational workflows.
- Gain practical exposure across manufacturing or QC/QA functions.
Eligibility / Qualifications
Accepted Courses
- 12th, ITI
- BSc, MSc
- BPharm, MPharm
- MBA (Marketing / General)
- BCom
- BTech, Diploma
Experience Requirements
- 1 to 8 years depending on department
- Apprenticeship roles require no prior experience
Location & Salary
Location: Damaira Pharmaceuticals Pvt. Ltd., SCO 321–322, Sector 35B, Chandigarh – 160022
Salary Range:
- Production Assistants: ₹2–3 LPA
- Manufacturing Executive: Based on experience
- RA: As per experience (3–8 yrs)
- BD: As per experience
- Microbiology / QC: ₹2–8 LPA
- QA: Based on 2–8 yrs experience
- Purchase: ₹2–6 LPA
- Apprenticeship: Stipend not specified
Application Process
Walk-in Interview Details
- Date: 21 December 2025
- Time: 10:00 AM – 4:30 PM
Venue: Damaira Pharmaceuticals Pvt. Ltd., SCO 321–322, Sector 35B, Chandigarh – 160022
Email: hr@damaira.com, hrd@damaira.com
Contact Numbers: +91 7341122753, +91 7341161560
FAQs
Are freshers eligible?
Yes, apprenticeship roles accept fresh graduates and diploma holders.
What departments have openings?
Production, QA, QC, Microbiology, Regulatory Affairs, Business Development, Purchase, and Apprenticeship.
What is the experience range for QC and Microbiology?
2–8 years depending on role.
What qualifications are required for Production roles?
12th/ITI for Assistant roles, BPharm for Executive roles.
Is MBA mandatory for Business Development?
Yes, MBA (Marketing) is required.
Summary Table
| Company | Damaira Pharmaceuticals Pvt. Ltd. |
|---|---|
| Vacancies | Production, RA, QA, QC, Micro, BD, Purchase, Apprenticeship |
| Required Education | 12th, ITI, BSc, MSc, BPharm, MPharm, MBA, BCom, BTech, Diploma |
| Experience | 0–8 years depending on role |
To apply for this job email your details to hr@damaira.com


