Emcure Walk-in QA, QC, Production, Packing & Engineering
- Company Overview
- Job Role & Responsibilities
- Quality Assurance (IPQA / APQR / Validation & Qualification) – Officer / Executive
- Production – Injectable (Aseptic Area) – Operator
- QC – Microbiology – Officer / Executive
- Packing / Visual Inspection – Officer / Operator
- Quality Control – Officer / Executive
- Engineering – Plant Maintenance / Utility – Officer / Technician
- Eligibility / Qualifications
- Relevant Courses
- Location & Salary
- Walk-In Details
- Application Process
- FAQs
- Is aseptic experience mandatory?
- Can freshers apply?
- What software experience helps for QA roles?
- Are ITI candidates eligible?
- Does the walk-in cover multiple departments?
- Summary Table
B.Pharm, M.Sc QA QC OSD & Injectable Openings | Mehsana
Emcure hiring B.Pharm, M.Sc, ITI candidates for QA, QC, Production, Packing & Engineering at Mehsana OSD & Injectable unit. Walk-in on 13 Dec.
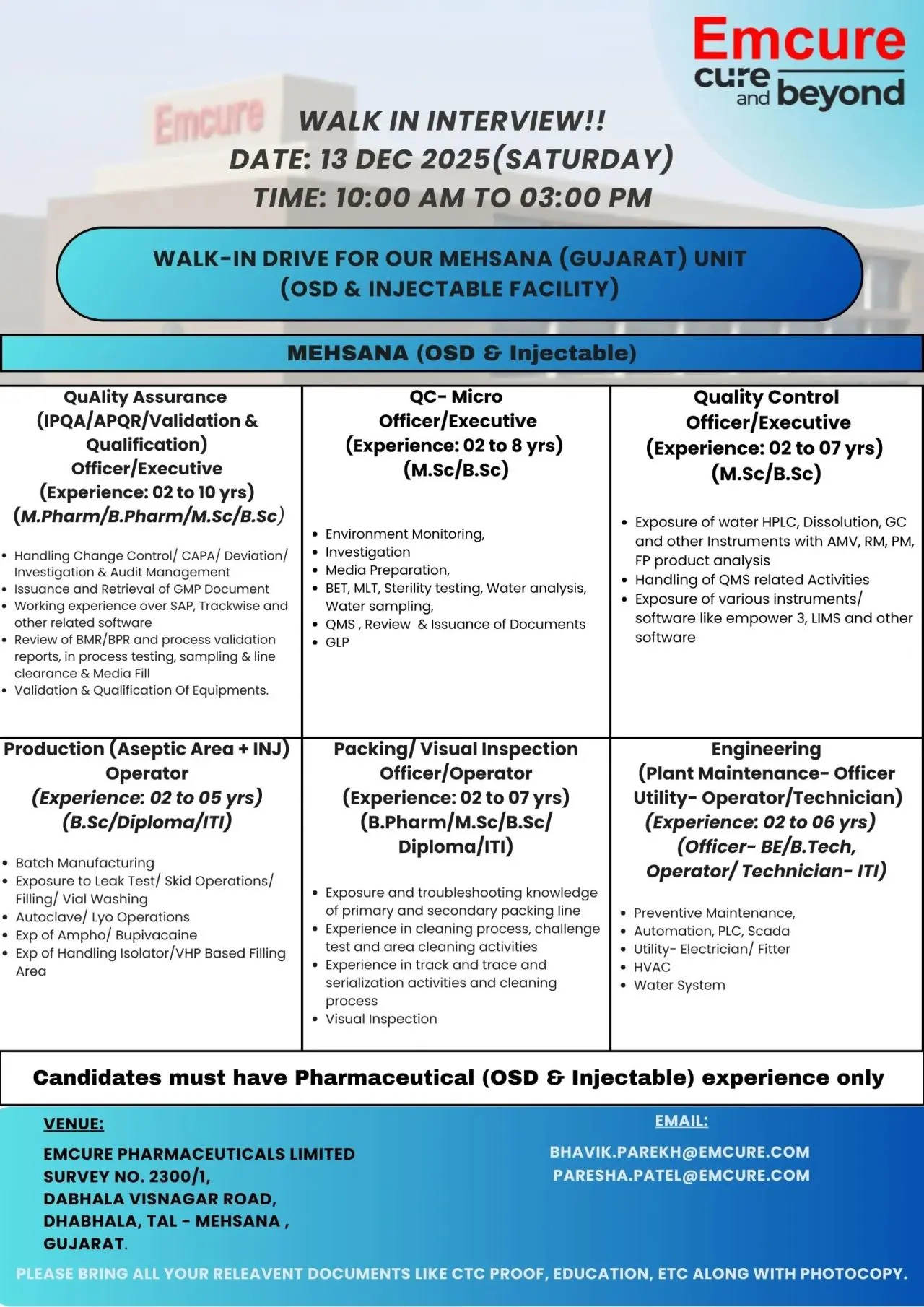
Emcure Pharmaceuticals is conducting a large-scale walk-in interview for its OSD and Injectable manufacturing facility in Mehsana, Gujarat. These positions are suited for experienced pharma professionals who have handled quality systems, aseptic operations, analytical testing, or engineering activities in regulated environments. The walk-in offers a direct pathway to join a well-established pharmaceutical organization with consistent growth and strong compliance systems.
Company Overview
Emcure Pharmaceuticals is a leading Indian multinational pharmaceutical company known for its portfolio across OSD, injectables, biologics, APIs, and critical care products. With a strong global presence and technologically advanced plants, Emcure operates on rigorous quality frameworks and regulatory expectations. The Mehsana unit handles both OSD and injectable operations, including aseptic manufacturing, validation, QC, and packaging.
Job Role & Responsibilities
Quality Assurance (IPQA / APQR / Validation & Qualification) – Officer / Executive
- Manage change control, CAPA, deviations, investigations, and audit readiness
- Handle issuance and retrieval of GMP documentation
- Review BMRs, DPRs, and process validation protocols
- Perform in-process checks, sampling, line clearance, and media fills
- Support qualification and validation of equipment and utilities
- Work on SAP, TrackWise, and related compliance software
Production – Injectable (Aseptic Area) – Operator
- Perform batch manufacturing activities for injectables
- Operate systems for leak testing, vial washing, skid operations, filling, autoclave, and lyophilization
- Work with isolators and VHP-based filling systems
- Handle specialized products such as Amphotericin and Bupivacaine
- Maintain aseptic behavior and strict GDP/GMP compliance
QC – Microbiology – Officer / Executive
- Conduct environmental monitoring and microbial investigations
- Execute sterility testing, MLT, BET, and water analysis
- Prepare media and maintain laboratory documentation
- Support GLP practices, QMS documentation, and instrument handling
Packing / Visual Inspection – Officer / Operator
- Work on both primary and secondary packing lines
- Perform cleaning, challenge tests, and line clearance
- Handle serialization, track-and-trace systems, and area hygiene activities
- Conduct visual inspection and maintain documentation accuracy
Quality Control – Officer / Executive
- Perform analysis using HPLC, GC, dissolution and critical analytical instruments
- Conduct RM, PM, FP testing with analytical method validation exposure
- Use software systems like Empower 3, LIMS, and other analytical tools
- Support QMS activities such as deviations and change controls
Engineering – Plant Maintenance / Utility – Officer / Technician
- Manage preventive maintenance, automation, PLC/SCADA operations
- Work on HVAC systems, utility operations, electrical and mechanical maintenance
- Handle water system maintenance and equipment troubleshooting
Eligibility / Qualifications
- QA: B.Pharm, M.Pharm, B.Sc, M.Sc with 2–10 years
- Production (Injectable): ITI, Diploma, B.Sc with 2–5 years
- QC Micro: B.Sc, M.Sc with 2–8 years
- Packing/Visual Inspection: ITI, Diploma, B.Sc, M.Sc, B.Pharm with 2–7 years
- QC: B.Sc, M.Sc with 2–7 years
- Engineering: ITI, Diploma, B.E, B.Tech with 2–6 years
Relevant Courses
B.Pharm, M.Pharm QA, M.Pharm Pharmaceutics, B.Sc/M.Sc Microbiology, B.Sc/M.Sc Chemistry, ITI Fitter, Diploma Mechanical/Electrical, B.Tech Mechanical/Electrical, PG Diploma in Aseptic Manufacturing.
Location & Salary
- Work Location: Emcure Pharmaceuticals Ltd., Mehsana (Gujarat)
- Salary: Based on experience; competitive within industry norms
- Note: Only candidates with OSD and injectable pharmaceutical experience are eligible
Walk-In Details
- Date: 13 December 2025 (Saturday)
- Time: 10:00 AM – 3:00 PM
- Venue: Emcure Pharmaceuticals Ltd., Survey No. 2300/1, Dabhala–Visnagar Road, Dhabhala, Tal-Mehsana, Gujarat
Application Process
Candidates unable to attend may share their resumes at:
Bring photocopies of:
- Educational certificates
- Experience letters
- Last 3 months salary slips
- CTC documents
- ID proof
FAQs
Is aseptic experience mandatory?
Yes, for injectable production and QC Micro roles.
Can freshers apply?
No. All roles require a minimum of 2 years of experience.
What software experience helps for QA roles?
TrackWise, SAP, and other quality management tools.
Are ITI candidates eligible?
Yes, ITI candidates may apply for production and engineering technician roles.
Does the walk-in cover multiple departments?
Yes, QA, QC, Production, Packing, and Engineering roles are all included.
Summary Table
| Company | Emcure Pharmaceuticals Ltd. |
|---|---|
| Vacancies | QA, QC, Production, Packing, Engineering |
| Required Education | ITI, Diploma, B.Sc, M.Sc, B.Pharm, M.Pharm, B.Tech |
| Experience | 2–10 years depending on role |