Aneta Pharmaceuticals Hiring QC, QA, RA, R&D

- Company Overview
- Job Role & Responsibilities
- Eligibility / Qualifications
- Relevant Courses
- Location & Salary
- Application Process
- FAQs
- Summary Table
Meta Title: Aneta Pharma QC, QA, RA, R&D Jobs for BPharm MSc | Ahmedabad
Meta Description: Aneta Pharmaceuticals hiring QC, QA Validation, Regulatory Affairs, R&D professionals in Ahmedabad. Multiple vacancies for B.Pharm, M.Pharm, MSc.
URL Slug: aneta-pharmaceuticals-osd-quality-control-qa-ra-rd
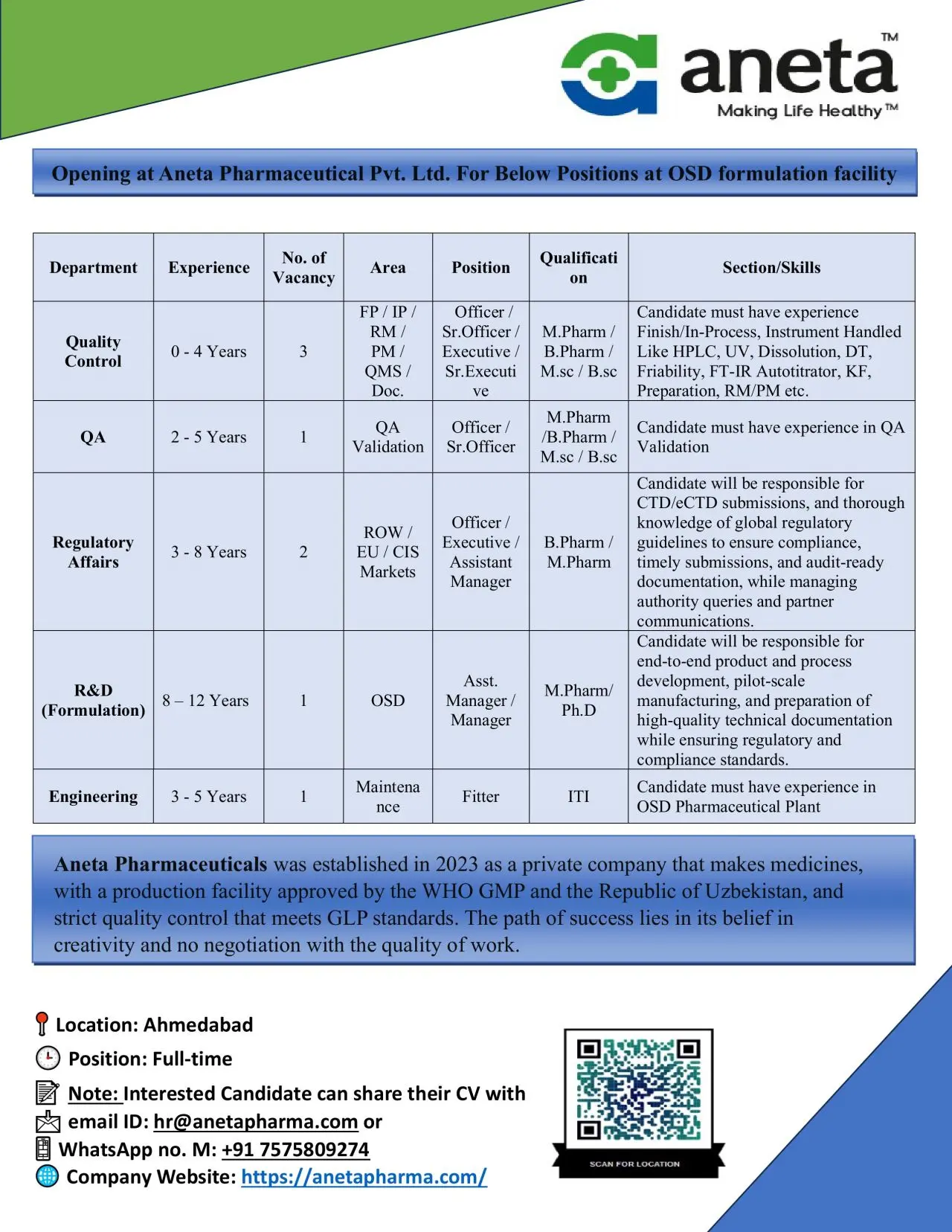
Aneta Pharmaceuticals Pvt. Ltd. is hiring skilled professionals for its Oral Solid Dosage (OSD) formulation facility in Ahmedabad. The company is expanding its quality, regulatory, research, and engineering teams and is inviting qualified candidates who want to work in a WHO-GMP–approved manufacturing environment focused on global compliance and product excellence.
Company Overview
Established in 2023, Aneta Pharmaceuticals Pvt. Ltd. is a fast-growing pharmaceutical manufacturer committed to delivering high-quality medicines. The company operates a WHO-GMP–approved production facility with regulatory approval from the Republic of Uzbekistan and follows strict GLP-aligned quality systems. Aneta’s culture emphasizes innovation, regulatory discipline, and uncompromised quality across all operations.
Job Role & Responsibilities
Aneta Pharmaceuticals is hiring across multiple departments at its OSD formulation plant.
Quality Control (QC)
Experience: 0–4 years
Vacancies: 3
Positions: Officer / Sr. Officer / Executive / Sr. Executive
Qualification: M.Pharm, B.Pharm, M.Sc, B.Sc
Responsibilities:
- Testing of finished products, in-process samples, raw materials, and packing materials
- Operation of analytical instruments including HPLC, UV, Dissolution, DT, FT-IR, Autotitrator, KF
- Performing friability and routine chemical analysis
- Preparation and review of QC documentation, specifications, and reports
- Supporting sampling, analysis, and release activities
QA Validation
Experience: 2–5 years
Vacancies: 1
Positions: Officer / Sr. Officer
Qualification: M.Pharm, B.Pharm, M.Sc, B.Sc
Responsibilities:
- Execution of validation activities for OSD products
- Preparation of validation protocols and reports
- Ensuring compliance with GMP, GLP, and regulatory standards
- Supporting audits and qualification activities
Regulatory Affairs (RA)
Experience: 3–8 years
Vacancies: 2
Positions: Officer / Executive / Assistant Manager
Qualification: B.Pharm / M.Pharm
Responsibilities:
- Preparation and submission of CTD and eCTD dossiers
- Handling regulatory submissions for ROW, EU, and CIS markets
- Managing authority queries and regulatory correspondence
- Maintaining audit-ready regulatory documentation
R&D – Formulation Development
Experience: 8–12 years
Vacancies: 1
Positions: Assistant Manager / Manager
Qualification: M.Pharm / Ph.D
Responsibilities:
- End-to-end development of OSD formulations
- Pilot-scale manufacturing and scale-up activities
- Preparation of technical documents, development reports, and protocols
- Supporting technology transfer to manufacturing
Engineering – Maintenance Fitter
Experience: 3–5 years
Vacancies: 1
Qualification: ITI / Diploma (Mechanical preferred)
Responsibilities:
- Maintenance and troubleshooting of OSD manufacturing equipment
- Preventive and breakdown maintenance activities
- Ensuring smooth operation of plant machinery and utilities
Eligibility / Qualifications
- Degrees accepted: B.Pharm, M.Pharm, B.Sc, M.Sc, Ph.D, ITI, Diploma
- Relevant experience in OSD pharmaceutical manufacturing is mandatory
- Strong understanding of GMP, GLP, documentation, and compliance systems
Relevant Courses
B.Pharm, M.Pharm Pharmaceutics, M.Sc Analytical Chemistry, M.Sc Chemistry, B.Sc Chemistry, Ph.D Pharmaceutics, ITI Mechanical, Diploma Mechanical Engineering.
Location & Salary
Job Location: Ahmedabad, Gujarat
Employment Type: Full-time
Salary will be offered based on experience, technical competency, and interview performance.
Application Process
Interested candidates can apply by sharing their updated CV via:
- Email: hr@anetapharma.com
- WhatsApp: +91 75758 09274
Company Website: https://anetapharma.com/
FAQs
1. Is OSD experience mandatory?
Yes, prior experience in OSD formulation plants is required for all roles.
2. Are freshers eligible for QC roles?
Yes, candidates with 0–4 years of experience can apply for QC positions.
3. Which regulatory markets does the RA team handle?
ROW, EU, and CIS markets.
4. What instruments should QC candidates be familiar with?
HPLC, UV, Dissolution, DT, FT-IR, KF, Autotitrator.
5. Where is the facility located?
Ahmedabad, Gujarat.
Summary Table
Company Aneta Pharmaceuticals Pvt. Ltd.
Vacancies QC (3), QA (1), RA (2), R&D (1), Engineering (1)
Required Education B.Pharm, M.Pharm, B.Sc, M.Sc, Ph.D, ITI/Diploma Experience 0-12 years depending on role
You must sign in to apply for this position.


