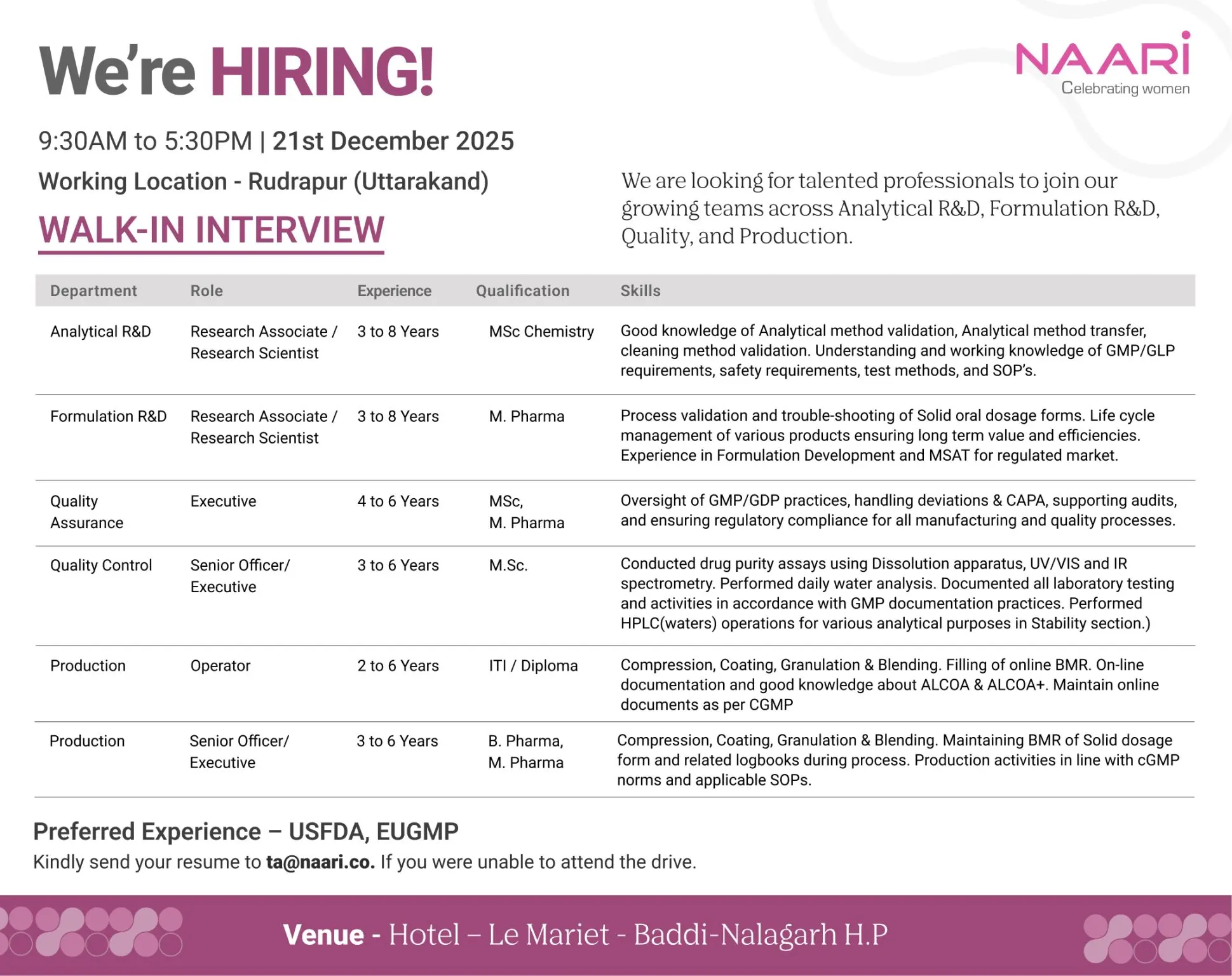
NAARI Walk-in R&D, QA, QC, Production

- Company Overview
- Job Role & Responsibilities
- Analytical R&D – Research Associate / Research Scientist
- Formulation R&D – Research Associate / Research Scientist
- Quality Assurance – Executive
- Quality Control – Senior Officer / Executive
- Production – Operator
- Production – Senior Officer / Executive
- Eligibility / Qualifications
- Relevant Courses
- Location & Salary
- Walk-in Interview Details
- Application Process
- FAQs
- Summary Table
MSc MPharm ITI Jobs at NAARI Pharma Rudrapur | Walk-in
NAARI Pharma hiring R&D, QA, QC, Production professionals in Rudrapur. Multiple vacancies for MSc, M.Pharm, B.Pharm, ITI.
NAARI, a growing pharmaceutical organization known for its inclusive culture and regulated manufacturing standards, is conducting a walk-in interview to strengthen its R&D, Quality, and Production teams. This hiring drive is ideal for experienced professionals seeking long-term growth in a compliant, innovation-driven pharma environment supporting regulated markets.
Company Overview
NAARI is a professionally managed pharmaceutical company focused on quality-driven development and manufacturing of solid oral dosage forms. With a strong emphasis on regulatory compliance, process excellence, and people-first culture, NAARI continues to expand its operations across research, quality systems, and manufacturing. The organization actively supports GMP, GLP, and global regulatory expectations, making it a strong workplace for professionals aiming to build sustainable pharma careers.
Job Role & Responsibilities
Analytical R&D – Research Associate / Research Scientist
Experience: 3–8 Years
Qualification: M.Sc Chemistry
Key Responsibilities:
- Analytical method development and validation for drug products
- Analytical method transfer and cleaning validation activities
- Preparation and review of protocols, reports, and SOPs
- Ensuring compliance with GMP and GLP requirements
- Supporting regulatory submissions and audits
Formulation R&D – Research Associate / Research Scientist
Experience: 3–8 Years
Qualification: M.Pharm (Pharmaceutics)
Key Responsibilities:
- Formulation development of solid oral dosage forms for regulated markets
- Process validation, scale-up, and troubleshooting activities
- Lifecycle management of products to ensure long-term efficiency
- MSAT support and technology transfer documentation
- Collaboration with Analytical, QA, and Production teams
Quality Assurance – Executive
Experience: 4–6 Years
Qualification: M.Sc / M.Pharm
Key Responsibilities:
- Oversight of GMP and GDP compliance across departments
- Handling deviations, CAPA, change control, and investigations
- Supporting internal and external regulatory audits
- Ensuring compliance with SOPs and quality systems
Quality Control – Senior Officer / Executive
Experience: 3–6 Years
Qualification: M.Sc
Key Responsibilities:
- Performing drug purity and dissolution testing
- Analysis using UV/VIS, IR, and HPLC (Waters)
- Stability studies and daily water analysis
- Maintaining laboratory documentation as per cGMP
Production – Operator
Experience: 2–6 Years
Qualification: ITI / Diploma
Key Responsibilities:
- Operation of compression, coating, granulation, and blending processes
- Online BMR filling and documentation
- Compliance with ALCOA and ALCOA+ principles
- Adherence to cGMP and SOP requirements
Production – Senior Officer / Executive
Experience: 3–6 Years
Qualification: B.Pharm / M.Pharm
Key Responsibilities:
- Supervising solid oral dosage manufacturing activities
- Maintaining BMRs and equipment logbooks
- Ensuring production activities meet cGMP norms
- Supporting audits and validation activities
Eligibility / Qualifications
- Educational background: M.Sc Chemistry, M.Pharm, B.Pharm, ITI, Diploma
- Prior experience in regulated pharmaceutical manufacturing preferred
- Exposure to USFDA and EU-GMP environments is an advantage
Relevant Courses
M.Sc Chemistry, M.Pharm Pharmaceutics, B.Pharm, Diploma in Pharmacy, ITI Mechanical / Production.
Location & Salary
Work Location: Rudrapur, Uttarakhand
Employment Type: Full-time
Salary will be offered as per industry standards and commensurate with experience and role.
Walk-in Interview Details
Date: 21st December 2025
Time: 9:30 AM – 5:30 PM
Venue: Hotel Le Mariet, Baddi–Nalagarh, Himachal Pradesh
Candidates unable to attend the walk-in may share their CV at ta@naari.co.
Application Process
- Attend the walk-in interview with updated resume
- Shortlisted candidates will be contacted for further rounds
FAQs
1. Is experience in regulated markets mandatory?
Preferred, especially USFDA and EU-GMP exposure, but not mandatory for all roles.
2. Can candidates from outside Uttarakhand apply?
Yes, candidates from any location may apply.
3. Are both R&D and Production roles available?
Yes, openings are available across R&D, Quality, and Production departments.
4. Is this a permanent position?
Yes, all roles are full-time permanent positions.
Summary Table
Company NAARI Pharmaceuticals
Vacancies Multiple – R&D, QA, QC, Production
Required Education M.Sc, M.Pharm, B.Pharm, ITI, Diploma
Experience 2–8 Years depending on role
You must sign in to apply for this position.


