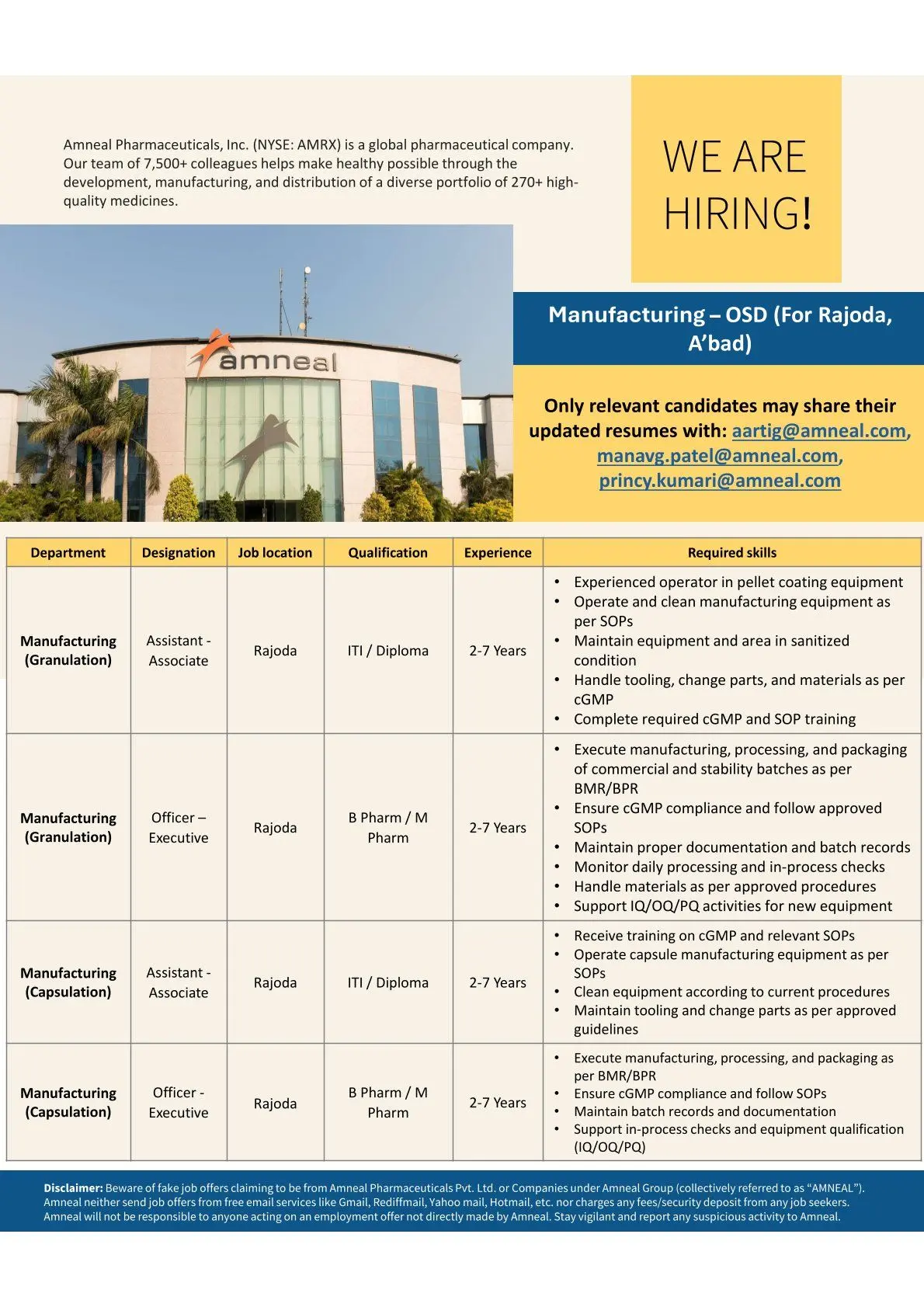
Amneal Hiring Manufacturing OSD

- Company Overview
- Job Role & Responsibilities
- Manufacturing – Granulation (Assistant / Associate)
- Manufacturing – Granulation (Officer / Executive)
- Manufacturing – Capsulation (Assistant / Associate)
- Manufacturing – Capsulation (Officer / Executive)
- Eligibility / Qualifications
- Location & Salary
- Application Process
- Why Build Your Career at Amneal Pharmaceuticals?
- Frequently Asked Questions (FAQs)
- Is this opportunity open to freshers?
- What type of manufacturing exposure is required?
- How can I apply for this position?
- Does Amneal charge any application or recruitment fees?
- Are these roles suitable for regulated market exposure?
- Important Disclaimer
- SEO Title Suggestions
- Eligible Education Courses
ITI, Diploma, B.Pharm Vacancies at Amneal – Rajoda
Amneal Pharmaceuticals hiring ITI, Diploma, B.Pharm candidates for Manufacturing OSD roles at Rajoda, Ahmedabad. Multiple vacancies.
Amneal Pharmaceuticals is hiring experienced professionals for its Manufacturing – Oral Solid Dosage (OSD) operations at Rajoda near Ahmedabad. This recruitment drive is aimed at skilled manufacturing operators, officers, and executives with hands-on exposure to granulation and capsulation processes in regulated pharmaceutical environments. Candidates with strong cGMP knowledge, equipment handling expertise, and batch manufacturing experience will find this an excellent opportunity to build a long-term career with a globally recognized pharmaceutical organization.
Company Overview
Amneal Pharmaceuticals, Inc. (NYSE: AMRX) is a global pharmaceutical company with a strong presence across the United States, India, and other international markets. With a workforce of over 7,500 professionals, Amneal develops, manufactures, and distributes more than 270 high-quality medicines spanning complex generics, specialty products, and biosimilars.
Amneal is widely respected for its compliance-driven manufacturing culture, advanced pharmaceutical technologies, and commitment to patient-centric healthcare solutions. The company operates world-class manufacturing facilities that adhere to global regulatory standards such as USFDA, WHO-GMP, and other international quality benchmarks. Working at Amneal offers exposure to regulated markets, structured training programs, and a stable professional growth path in pharmaceutical manufacturing.
Job Role & Responsibilities
Manufacturing – Granulation (Assistant / Associate)
Professionals in this role will support granulation operations within OSD manufacturing units. Key responsibilities include:
• Operating pellet coating and granulation equipment as per approved SOPs
• Cleaning and maintaining manufacturing equipment and production areas
• Ensuring sanitized conditions as per cGMP requirements
• Handling tooling, change parts, and raw materials correctly
• Executing manufacturing and processing activities as per BMR and BPR
• Completing mandatory cGMP and SOP training programs
• Supporting commercial and stability batch manufacturing
Manufacturing – Granulation (Officer / Executive)
This role involves supervisory and technical responsibilities within granulation operations. Responsibilities include:
• Ensuring strict adherence to cGMP guidelines and approved SOPs
• Maintaining accurate batch manufacturing records and documentation
• Monitoring daily production activities and in-process checks
• Handling materials as per approved procedures and inventory controls
• Supporting equipment qualification activities such as IQ, OQ, and PQ
• Operating capsule manufacturing equipment as per validated procedures
• Maintaining tooling and change parts according to approved guidelines
• Executing manufacturing, processing, and packaging activities efficiently
Manufacturing – Capsulation (Assistant / Associate)
Capsulation assistants and associates will be involved in day-to-day production operations. Key duties include:
• Operating capsulation equipment in compliance with SOPs
• Cleaning equipment and production areas as per current procedures
• Supporting in-process checks during manufacturing
• Maintaining batch records and production documentation
• Assisting senior staff during equipment setup and changeovers
Manufacturing – Capsulation (Officer / Executive)
Officers and executives in capsulation will oversee manufacturing processes and compliance activities, including:
• Ensuring cGMP compliance across capsulation operations
• Maintaining batch records, logs, and documentation
• Supporting in-process checks and process validation
• Assisting with IQ, OQ, and PQ activities for equipment
• Coordinating with quality and engineering teams to resolve deviations
Eligibility / Qualifications
The following educational qualifications are eligible for these roles:
ITI, Diploma in Pharmacy, Diploma in Mechanical Engineering, Diploma in Electrical Engineering, Diploma in Instrumentation, B.Pharm, M.Pharm
Candidates must have relevant pharmaceutical manufacturing experience ranging from 2 to 7 years. Prior exposure to OSD manufacturing, granulation, capsulation, and regulated plant environments is essential.
Location & Salary
Job Location:
• Rajoda, Ahmedabad (OSD Manufacturing Facility)
Salary will be offered as per industry standards and will depend on the candidate’s qualifications, experience, and current compensation structure. Amneal follows a transparent and performance-based compensation system.
Application Process
Interested and relevant candidates are requested to share their updated resumes via official company email IDs. Only shortlisted candidates will be contacted for further evaluation.
Apply via Email:
• aartig@amneal.com
• manavg.patel@amneal.com
• princy.kumari@amneal.com
Candidates should ensure that their resumes clearly highlight hands-on experience in pharmaceutical manufacturing, equipment operation, cGMP compliance, and batch documentation.
Why Build Your Career at Amneal Pharmaceuticals?
• Global pharmaceutical organization with NYSE listing
• Strong compliance and quality-driven culture
• Exposure to regulated markets and advanced technologies
• Structured training and career progression
• Contribution to affordable and quality healthcare solutions
Frequently Asked Questions (FAQs)
Is this opportunity open to freshers?
No. These roles require a minimum of 2 years of relevant pharmaceutical manufacturing experience.
What type of manufacturing exposure is required?
Candidates must have hands-on experience in OSD manufacturing, specifically granulation or capsulation processes.
How can I apply for this position?
Interested candidates should apply by sending their resumes to the official Amneal email IDs provided above.
Does Amneal charge any application or recruitment fees?
No. Amneal does not charge any fees or security deposits at any stage of the recruitment process.
Are these roles suitable for regulated market exposure?
Yes. Amneal operates regulated manufacturing facilities, offering strong exposure to compliance and audit standards.
Important Disclaimer
Beware of fake job offers claiming to represent Amneal Pharmaceuticals or its group companies. Amneal does not send job offers from free email services and does not charge any recruitment fees. Candidates are advised to apply only through official company communication channels.
SEO Title Suggestions
Amneal Pharmaceuticals Hiring Manufacturing OSD Staff – ITI to M.Pharm
Eligible Education Courses
ITI Fitter, ITI Mechanical, ITI Electrical, Diploma in Pharmacy, Diploma in Mechanical Engineering, Diploma in Electrical Engineering, Diploma in Instrumentation, B.Pharm, M.Pharm
| Company | Amneal Pharmaceuticals, Inc. |
|---|---|
| Vacancies | Manufacturing Assistant, Associate, Officer, Executive (Granulation, Capsulation) |
| Required Education | ITI, Diploma, B.Pharm, M.Pharm |
| Experience | 2–7 Years |
.
To apply for this job email your details to aartig@amneal.com


