Micro Hiring multiple QA

- Company Overview
- Job Role & Responsibilities
- QA – Documentation / QA-QMS
- QA – Media Fill / Validation
- Eligibility / Qualifications
- Educational Qualifications
- Experience Requirements
- Location & Salary
- Application Process
- Why Join Micro Labs Limited
- FAQs
- Who can apply for these QA roles?
- Is sterile manufacturing experience mandatory?
- What systems knowledge is preferred?
- Are these roles based only in Bangalore?
- SEO Optimized Title Suggestions
- Summary Table
Micro Labs B.Pharm QA Vacancies – Bangalore
Micro Labs Limited hiring B.Pharm, M.Pharm professionals for multiple QA vacancies at sterile facility, Bangalore.
Micro Labs Limited is hiring experienced Quality Assurance professionals for its sterile manufacturing facility at Bommasandra, Bangalore. This recruitment drive focuses on QA Documentation, QA-QMS, and QA Media Fill & Validation roles, offering strong career growth for professionals with exposure to regulated sterile pharmaceutical environments. These roles are ideal for candidates seeking stability, regulatory exposure, and long-term careers with one of India’s most trusted pharmaceutical companies.
Company Overview
Micro Labs Limited is a leading Indian pharmaceutical company with a strong presence in domestic and international markets. Known for its quality-driven culture, Micro Labs operates WHO-GMP compliant and globally regulated manufacturing facilities, including advanced sterile production units. The company’s commitment to patient safety, regulatory compliance, and continuous improvement has positioned it as a respected name in pharmaceutical manufacturing.
The sterile facility at Bommasandra is a critical manufacturing hub, supporting injectable and sterile dosage forms that meet stringent global regulatory standards. Working here provides professionals with hands-on exposure to audits, inspections, and advanced quality systems essential for modern pharmaceutical manufacturing.
Job Role & Responsibilities
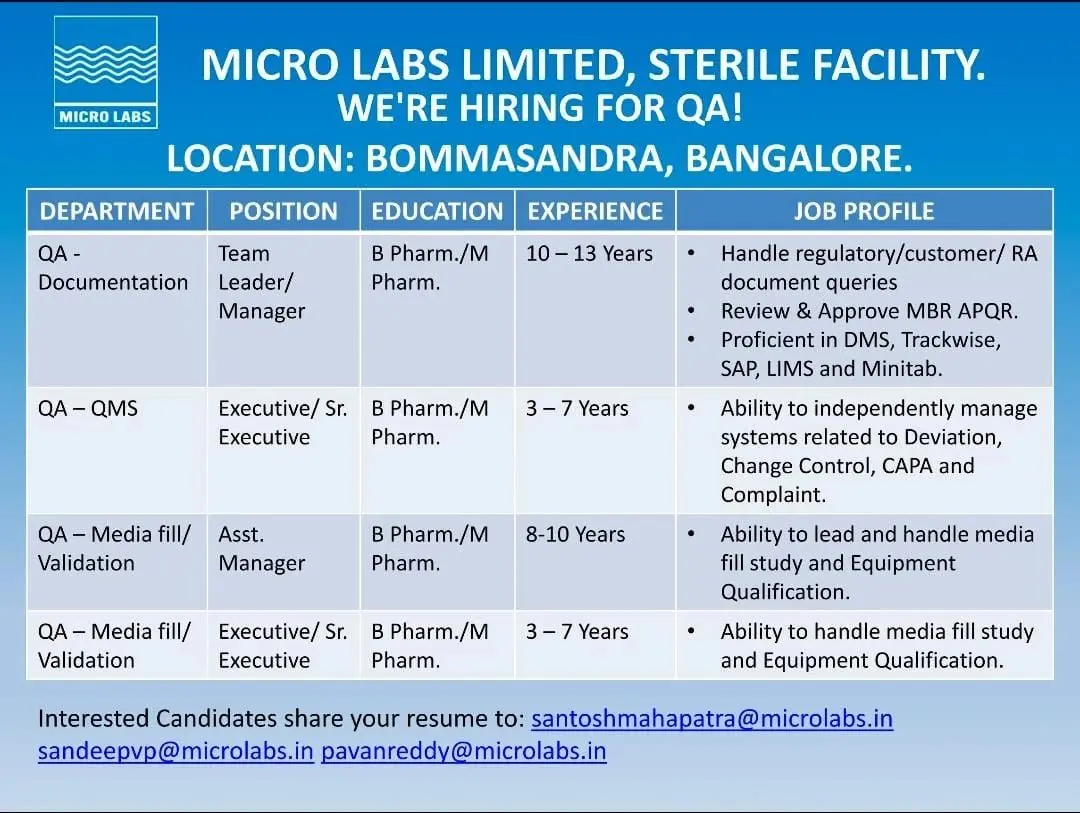
QA – Documentation / QA-QMS
Team Leader / Manager (10–13 Years)
Senior QA professionals in documentation and QMS roles will oversee quality systems and regulatory documentation activities.
Key responsibilities include:
- Handling regulatory authority, customer, and regulatory affairs documentation queries
- Review and approval of MBR, APQR, and quality system documents
- Oversight of quality management systems and continuous improvement initiatives
- Effective use of electronic systems such as DMS, TrackWise, SAP, LIMS, and Minitab
- Supporting internal, external, and regulatory audits
Executive / Senior Executive (3–7 Years)
Mid-level QA professionals will independently manage core quality systems.
Key responsibilities include:
- Management of deviations, change control, CAPA, and customer complaints
- Investigation and closure of quality events within defined timelines
- Documentation review and compliance monitoring
- Coordination with cross-functional teams to maintain GMP compliance
QA – Media Fill / Validation
Assistant Manager (8–10 Years)
Senior validation professionals will lead sterility assurance activities.
Key responsibilities include:
- Planning, execution, and review of media fill studies
- Leading equipment qualification and validation activities
- Ensuring compliance with sterile manufacturing regulations
- Documentation and reporting of validation activities
- Supporting regulatory inspections and audit responses
Executive / Senior Executive (3–7 Years)
Validation professionals will support and execute validation programs.
Key responsibilities include:
- Execution of media fill studies under sterile conditions
- Equipment qualification and requalification activities
- Preparation and review of validation protocols and reports
- Coordination with production and engineering teams
Eligibility / Qualifications
Educational Qualifications
- B.Pharm
- M.Pharm
Relevant courses include Pharmaceutics, Quality Assurance, Industrial Pharmacy, Pharmaceutical Technology, Regulatory Affairs, Validation Sciences, and related pharmaceutical disciplines.
Experience Requirements
- Executive / Senior Executive: 3 to 7 years
- Assistant Manager: 8 to 10 years
- Team Leader / Manager: 10 to 13 years
Candidates must have experience in sterile pharmaceutical manufacturing environments.
Location & Salary
- Job Location: Bommasandra, Bangalore, Karnataka
Salary will be offered as per industry standards and will be commensurate with qualification, experience, and role level. Micro Labs offers competitive compensation, structured growth, and long-term career stability.
Application Process
Interested and eligible candidates can apply by sharing their updated resumes via official email IDs:
Shortlisted candidates will be contacted for further interview rounds.
Why Join Micro Labs Limited
- Reputed pharmaceutical organization with global regulatory exposure
- Advanced sterile manufacturing facility
- Strong quality culture and compliance-driven operations
- Long-term career growth in QA, QMS, and validation
- Direct contribution to patient safety and healthcare advancement
FAQs
Who can apply for these QA roles?
Candidates with B.Pharm or M.Pharm qualifications and relevant sterile QA experience can apply.
Is sterile manufacturing experience mandatory?
Yes. Prior experience in sterile or injectable manufacturing environments is essential.
What systems knowledge is preferred?
Hands-on experience with DMS, TrackWise, SAP, LIMS, and statistical tools like Minitab is preferred.
Are these roles based only in Bangalore?
Yes. All positions are based at the Bommasandra sterile facility in Bangalore.
SEO Optimized Title Suggestions
- Micro Labs Limited Hiring QA Professionals – Bangalore
- Micro Labs QA Documentation & Validation Jobs
- Micro Labs Sterile Facility QA Vacancies
Summary Table
| Company | Micro Labs Limited |
|---|---|
| Vacancies | QA Team Leader, QA Manager, QA Assistant Manager, QA Executive, Senior Executive |
| Required Education | B.Pharm, M.Pharm |
| Experience | 3 to 13 years |
To apply for this job email your details to santoshmahapatra@microlabs.in


