Ami Lifesciences Walk-in API QC & QA

- Company Overview
- Job Role & Responsibilities
- Quality Control – API
- Quality Assurance – API
- Eligibility / Qualifications
- Educational Qualifications
- Experience Requirements
- Required Skills
- Location & Salary
- Walk-In Interview Details
- Application Process
- Important Notice
- Why Join Ami Lifesciences
- FAQs
- Who can attend this walk-in interview?
- Is API experience mandatory?
- Are freshers eligible?
- Is there any application or interview fee?
- Summary Table
MSc MPharma API QC QA Jobs in Vadodara – Ami Lifesciences
Ami Lifesciences hiring API QC & QA professionals in Vadodara. MSc, MPharma eligible. 2–8 yrs experience. Walk-in interview.
Ami Lifesciences is conducting a walk-in interview for experienced Quality Control and Quality Assurance professionals at its API manufacturing operations near Vadodara, Gujarat. This recruitment drive is aimed at chemistry and pharmacy graduates with proven exposure to API environments who want to work in a regulated, audit-ready pharmaceutical setup.
Quality functions in API manufacturing sit at the core of patient safety and regulatory trust. These roles directly influence product purity, data integrity, and global compliance. At Ami Lifesciences, QC and QA teams work with advanced analytical systems, robust quality frameworks, and continuous improvement programs aligned with international regulatory expectations.
Company Overview
Ami Lifesciences is a recognized pharmaceutical company operating in the Active Pharmaceutical Ingredient (API) segment. The organization is known for its strong compliance culture, quality-first mindset, and consistent performance in regulated markets. Its API operations near Vadodara follow stringent cGMP requirements and are designed to meet the expectations of global customers and regulators.
The company emphasizes data integrity, process discipline, and technology-driven quality systems. Professionals joining Ami Lifesciences gain exposure to audits, inspections, and quality practices that strengthen long-term careers in API quality control and quality assurance.
Job Role & Responsibilities
Quality Control – API
Quality Control professionals will handle analytical testing and laboratory compliance activities in an API setting.
Key Responsibilities:
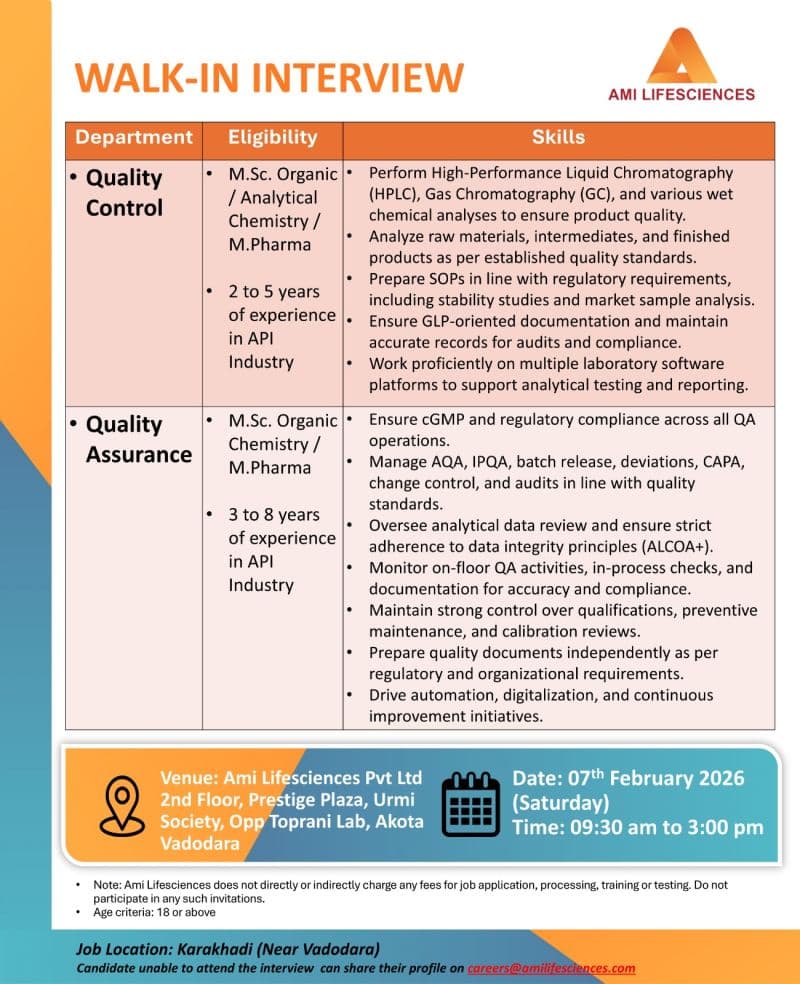
- Performing HPLC, GC, and wet chemical analysis to verify product quality
- Analysis of raw materials, intermediates, and finished API products
- Preparation and review of SOPs as per regulatory and internal requirements
- Conducting stability studies and market sample analysis
- Maintaining GLP-compliant documentation and laboratory records
- Working on laboratory software platforms for data analysis and reporting
- Supporting internal audits and regulatory inspections
These responsibilities align with high-demand API quality control jobs requiring strong analytical expertise.
Quality Assurance – API
Quality Assurance professionals will oversee compliance, documentation, and quality systems across API manufacturing operations.
Key Responsibilities:
- Ensuring cGMP and regulatory compliance across QA functions
- Handling AQA, IPQA, batch release, deviations, CAPA, and change control
- Managing internal, customer, and regulatory audits
- Reviewing analytical data with strict adherence to ALCOA+ data integrity principles
- Monitoring on-floor QA activities and in-process checks
- Reviewing qualification, calibration, and preventive maintenance records
- Preparing quality documents independently as per regulatory expectations
- Driving automation, digitalization, and continuous improvement initiatives
This role is ideal for candidates targeting senior API QA jobs with audit and compliance exposure.
Eligibility / Qualifications
Educational Qualifications
- M.Sc. Organic Chemistry
- M.Sc. Analytical Chemistry
- M.Pharmacy
(Relevant courses: MSc Organic Chemistry, MSc Analytical Chemistry, MPharm – Pharmaceutical Analysis, Quality Assurance)
Experience Requirements
- Quality Control: 2 to 5 years of experience in API industry
- Quality Assurance: 3 to 8 years of experience in API industry
Required Skills
- Strong knowledge of cGMP regulations and API quality systems
- Hands-on experience with HPLC and GC instrumentation
- Understanding of data integrity and ALCOA+ principles
- Documentation accuracy and audit readiness mindset
- Ability to work effectively in regulated API manufacturing environments
These skills are closely aligned with high-CPC pharmaceutical API jobs and compliance-focused roles.
Location & Salary
Job Location: Karakhadi (Near Vadodara), Gujarat
Salary: Compensation will be competitive and aligned with industry standards. Final salary will depend on experience, technical expertise, and interview performance. Ami Lifesciences offers stable growth and long-term career development in API quality roles.
Walk-In Interview Details
- Date: 07th February 2026 (Saturday)
- Time: 09:30 AM to 03:00 PM
- Venue: Ami Lifesciences Pvt. Ltd., 2nd Floor, Prestige Plaza, Urmi Society, Opposite Toprani Lab, Akota, Vadodara
- Age Criteria: 18 years and above
Candidates should carry updated resumes, academic certificates, experience documents, and valid identification.
Application Process
Candidates unable to attend the walk-in interview may apply by sharing their updated profile via email.
Apply via Email: careers@amilifesciences.com
Applicants are advised to mention the department applied for (QC or QA) in the email subject line for faster processing.
Important Notice
Ami Lifesciences does not directly or indirectly charge any fees for job applications, processing, training, or testing. Candidates are advised not to respond to fraudulent job offers.
Why Join Ami Lifesciences
- Strong reputation in the API pharmaceutical segment
- Exposure to global quality and regulatory systems
- Advanced analytical and QA infrastructure
- Emphasis on data integrity and continuous improvement
- Direct contribution to safe and compliant healthcare products
These roles are well suited for candidates searching for API QC jobs in Vadodara, API QA careers, pharmaceutical quality assurance roles, and GMP compliance jobs.
FAQs
Who can attend this walk-in interview?
Candidates with MSc or MPharma qualifications and relevant API industry experience can attend.
Is API experience mandatory?
Yes. Prior experience in the API industry is mandatory for both QC and QA positions.
Are freshers eligible?
No. These roles require prior hands-on experience in API quality functions.
Is there any application or interview fee?
No. Ami Lifesciences does not charge any fees at any stage of recruitment.
Summary Table
| Company | Ami Lifesciences |
|---|---|
| Vacancies | Quality Control Executive, Quality Assurance Executive |
| Required Education | M.Sc. Organic/Analytical Chemistry, M.Pharm |
| Experience | 2–5 years (QC), 3–8 years (QA) |
To apply for this job email your details to careers@amilifesciences.com


