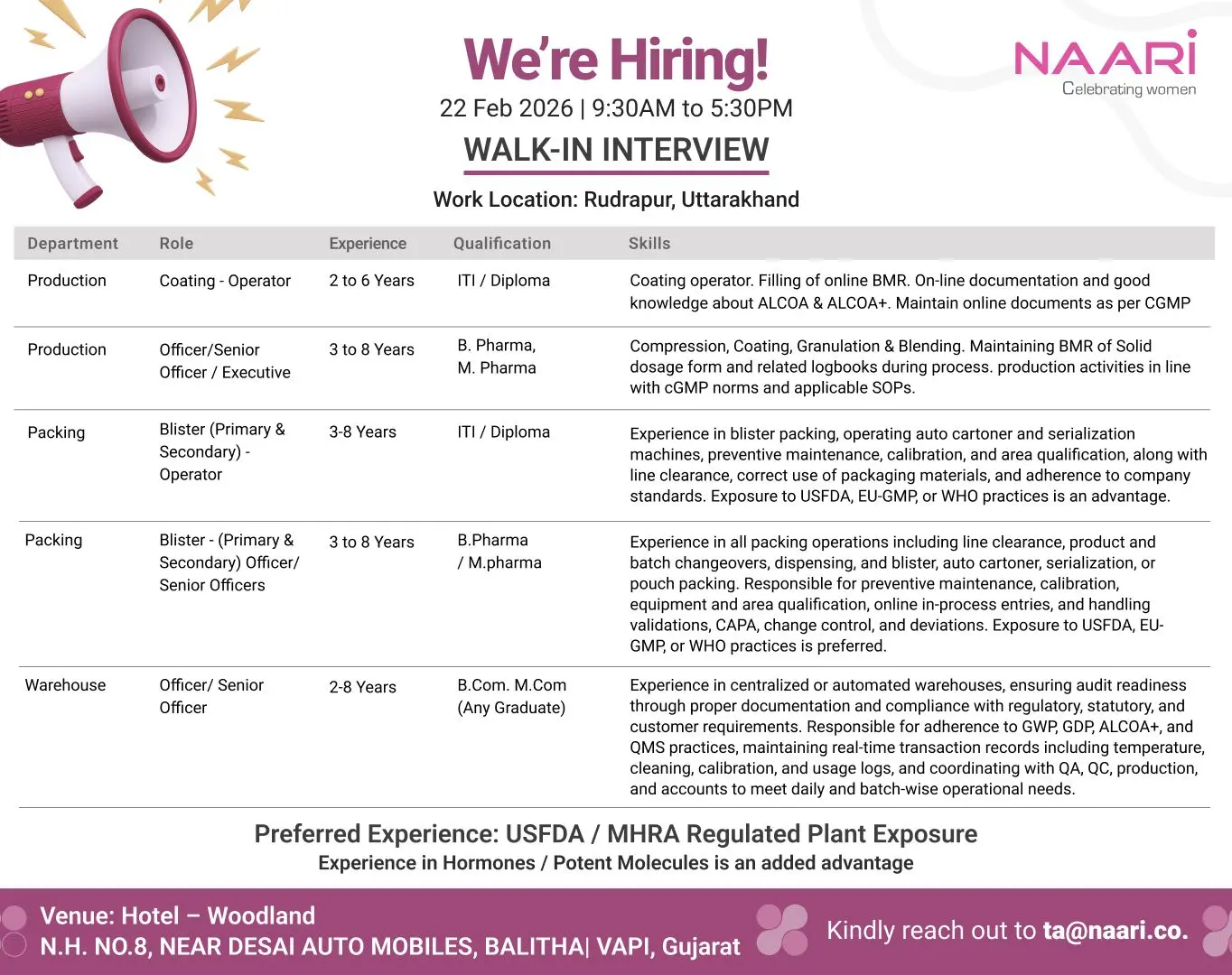
Naari walk‑in Production, Packing & Warehouse

- Company Overview
- Job Role & Responsibilities
- Production – Coating Operator
- Production – Officer / Senior Officer / Executive
- Packing – Blister Operator (Primary & Secondary)
- Packing – Officer / Senior Officer
- Warehouse – Officer / Senior Officer
- Preferred Experience
- Eligibility / Qualifications
- Eligible Educational Qualifications (comma‑separated)
- Location & Interview Details
- Application Process
- Why Consider a Career at Naari
- Frequently Asked Questions (FAQs)
- Who can attend the walk‑in interview?
- Is regulated plant exposure mandatory?
- Are hormone or potent molecule experience candidates preferred?
- What documents should be carried?
- Summary Table
ITI BPharm Pharma Walk‑In | Production & Packing | Rudrapur
Naari Pharma walk‑in for Production, Packing & Warehouse roles in Rudrapur. ITI, Diploma, BPharm, MPharm, BCom eligible.
Naari is conducting a walk‑in interview drive for multiple positions across Production, Packing, and Warehouse departments for its pharmaceutical manufacturing operations in Rudrapur, Uttarakhand. This hiring initiative targets experienced professionals from regulated formulation plants who are familiar with cGMP practices, documentation discipline, and audit‑ready manufacturing environments.
Candidates with exposure to USFDA, EU‑GMP, MHRA, or WHO‑compliant facilities will find this opportunity particularly aligned with their experience. If you are searching for pharma walk‑in interviews, production operator jobs, blister packing vacancies, or warehouse officer roles in regulated plants, this recruitment drive offers strong growth potential.
Company Overview
Naari is a pharmaceutical organization known for its focus on specialized formulations and compliance‑driven manufacturing systems. The company operates with strong quality governance, data integrity standards, and regulatory alignment to serve domestic and international healthcare markets.
The manufacturing setup emphasizes adherence to cGMP, ALCOA and ALCOA+ data integrity principles, validated processes, and structured quality management systems. Professionals working at Naari gain exposure to high‑compliance production environments, documentation excellence, and cross‑functional coordination across QA, QC, Production, Engineering, and Supply Chain.
Job Role & Responsibilities
Naari is hiring across the following departments:
Production – Coating Operator
Experience: 2 to 6 Years
Qualification: ITI / Diploma
Key Responsibilities:
- Operate coating equipment for solid dosage forms
- Fill and maintain online BMR entries accurately
- Ensure on‑line documentation compliance as per cGMP norms
- Maintain ALCOA & ALCOA+ data integrity practices
- Coordinate with QA during in‑process checks
Production – Officer / Senior Officer / Executive
Experience: 3 to 8 Years
Qualification: B.Pharm, M.Pharm
Key Responsibilities:
- Handle Compression, Coating, Granulation, and Blending operations
- Maintain BMR and production logbooks during processing
- Ensure activities comply with cGMP norms and approved SOPs
- Support validation batches and process documentation
- Coordinate with QA, QC, and Engineering during operations
Packing – Blister Operator (Primary & Secondary)
Experience: 3 to 8 Years
Qualification: ITI / Diploma
Key Responsibilities:
- Operate blister packing and auto cartoner machines
- Manage serialization processes and preventive maintenance
- Perform calibration checks and line clearance
- Ensure correct use of packaging materials
- Maintain compliance with USFDA, EU‑GMP, or WHO standards
Packing – Officer / Senior Officer
Experience: 3 to 8 Years
Qualification: B.Pharm, M.Pharm
Key Responsibilities:
- Oversee primary and secondary packing operations
- Manage product changeovers and dispensing activities
- Handle CAPA, deviations, change control, and validations
- Maintain online in‑process documentation
- Ensure equipment qualification and area compliance
Warehouse – Officer / Senior Officer
Experience: 2 to 8 Years
Qualification: B.Com, M.Com, Any Graduate
Key Responsibilities:
- Manage centralized or automated warehouse operations
- Maintain real‑time transaction and temperature logs
- Ensure adherence to GWP, GDP, ALCOA+, and QMS standards
- Support audit readiness through accurate documentation
- Coordinate with QA, QC, Production, and Accounts teams
Preferred Experience
- Exposure to USFDA / MHRA regulated plants
- Experience handling Hormones or Potent Molecules (added advantage)
Eligibility / Qualifications
Eligible Educational Qualifications (comma‑separated)
ITI, Diploma, B.Pharm, M.Pharm, B.Com, M.Com, Any Graduate
Candidates must have prior pharmaceutical formulation manufacturing exposure relevant to the applied department.
Location & Interview Details
Work Location: Rudrapur, Uttarakhand
Walk‑In Date: 22 February 2026
Interview Time: 9:30 AM to 5:30 PM
Walk‑In Venue:
Hotel Woodland
N.H. No. 8, Near Desai Automobiles, Balitha, Vapi, Gujarat
Application Process
Interested candidates may attend the walk‑in interview directly or share their updated resume in advance.
Email ID: ta@naari.co
Candidates are advised to carry updated resume, educational documents, experience certificates, and relevant compliance records.
Why Consider a Career at Naari
- Exposure to regulated pharmaceutical manufacturing
- Strong focus on cGMP, data integrity, and compliance systems
- Opportunities in solid dosage production and advanced packing lines
- Experience in serialization and global regulatory practices
- Long‑term growth in audit‑ready manufacturing environments
These roles are suitable for professionals seeking pharmaceutical production jobs, blister packing operator vacancies, warehouse officer roles in pharma companies, and GMP‑compliant manufacturing careers.
Frequently Asked Questions (FAQs)
Who can attend the walk‑in interview?
Candidates with relevant pharma manufacturing experience and required qualifications can attend.
Is regulated plant exposure mandatory?
It is preferred, especially exposure to USFDA, MHRA, or EU‑GMP facilities.
Are hormone or potent molecule experience candidates preferred?
Yes. Experience in hormones or potent molecule facilities is considered an advantage.
What documents should be carried?
Updated CV, educational certificates, experience letters, and compliance‑related documents.
Summary Table
| Company | Naari |
|---|---|
| Vacancies | Production Operator, Production Officer, Packing Operator, Packing Officer, Warehouse Officer |
| Required Education | ITI, Diploma, B.Pharm, M.Pharm, B.Com, M.Com |
| Experience | 2–8 Years (Role‑specific, Regulated Plant Preferred) |
To apply for this job email your details to ta@naari.co


