Macleods Walk-in QC, QA, Production & Engineering

- Company Overview
- Walk-In Drive Details
- Job Role & Responsibilities
- Quality Control (OSD / API)
- Quality Control (Injectables)
- Quality Assurance (OSD)
- Production (OSD)
- Engineering Services (OSD / API)
- Eligibility / Qualifications
- Location & Salary
- Application Process
- Why Consider Macleods Indore?
- Frequently Asked Questions (FAQs)
- Summary
MPharm/BPharm QC QA Vacancies Indore
M.Sc, B.Pharm, M.Pharm vacancies at Macleods Indore plant. Walk-in on 22 Feb 2026 for QC, QA, Production & Engineering roles.
Macleods Pharmaceuticals is conducting a large-scale walk-in drive for its Indore manufacturing plant covering Quality Control, Quality Assurance, Production, and Engineering Services across OSD, API, and Injectable facilities. Professionals with 2–13 years of pharmaceutical manufacturing experience in regulated environments are invited to participate.
This hiring drive is focused on high-demand pharma roles such as QC HPLC analyst, QA QMS executive, IPQA officer, OSD production officer, injectable QC reviewer, plant maintenance engineer, and HVAC pharma technician in Madhya Pradesh.
Company Overview
Macleods Pharmaceuticals is one of India’s fastest-growing pharmaceutical companies with a strong global presence across regulated and semi-regulated markets. The company operates USFDA-approved manufacturing facilities and is known for robust compliance systems, strong quality culture, and export-driven operations.
The Indore plant supports Oral Solid Dosage (OSD), API, and sterile manufacturing operations. With strong regulatory frameworks aligned to cGMP, data integrity compliance, process validation, and audit readiness systems, Macleods offers professionals exposure to international pharmaceutical standards.
Working at Macleods provides hands-on involvement in validation protocols, regulatory documentation, deviation management, CAPA systems, stability studies, environmental monitoring, and advanced pharmaceutical manufacturing technologies.
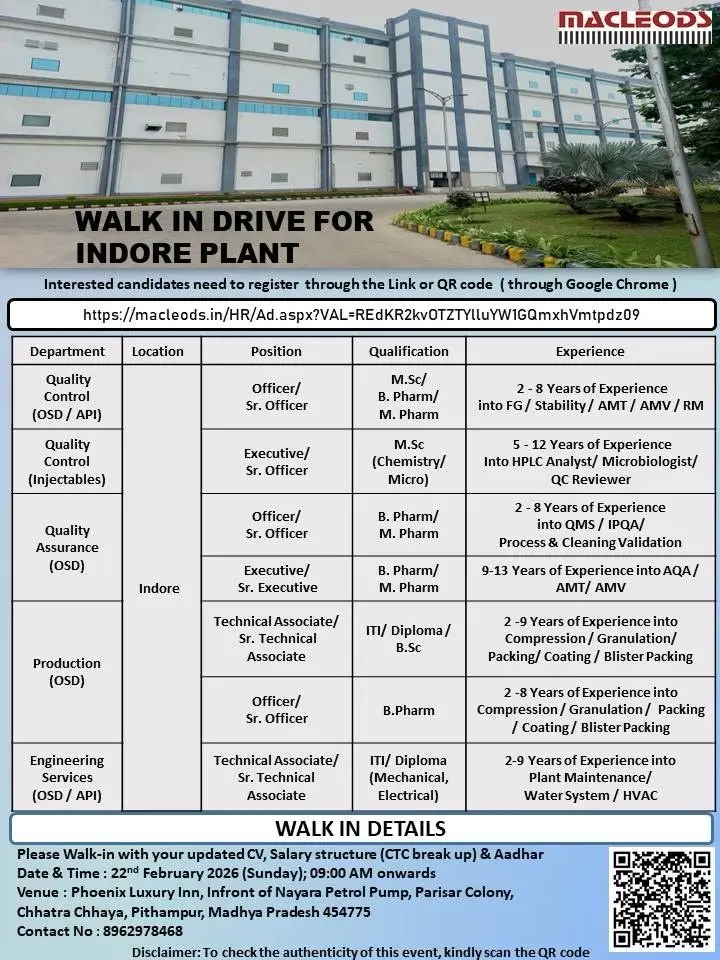
Walk-In Drive Details
Date: 22 February 2026 (Sunday)
Time: 09:00 AM onwards
Venue: Phoenix Luxury Inn, In front of Nayara Petrol Pump, Parisar Colony, Chhatra Chhaya, Pithampur, Madhya Pradesh – 454775
Location: Indore, Madhya Pradesh
Contact No: 8962978468
Candidates are required to register through the official registration link before attending the walk-in.
Job Role & Responsibilities
Quality Control (OSD / API)
Designation: Officer / Sr. Officer
Qualification: M.Sc, B.Pharm, M.Pharm
Experience: 2–8 Years (FG / Stability / AMT / AMV / RM)
Key Responsibilities:
- Perform finished goods and raw material analysis.
- Conduct analytical method validation (AMV) and analytical method transfer (AMT).
- Operate HPLC, GC, UV, dissolution and other analytical instruments.
- Maintain stability studies and regulatory documentation.
- Ensure compliance with GLP and data integrity guidelines.
High CPC keywords: HPLC analyst jobs, stability testing pharma, analytical method validation, QC executive vacancy.
Quality Control (Injectables)
Designation: Executive / Sr. Officer
Qualification: M.Sc (Chemistry / Microbiology)
Experience: 5–12 Years (QC Reviewer / HPLC Analyst / Microbiologist)
Key Responsibilities:
- Review analytical data and QC documentation.
- Conduct sterility testing and microbiological analysis.
- Perform environmental monitoring and water analysis.
- Handle regulatory audit queries and compliance documentation.
Preferred exposure: sterile injectable QC, microbiology lab operations, USFDA audit support.
Quality Assurance (OSD)
Designation: Officer / Sr. Officer / Executive / Sr. Executive
Qualification: B.Pharm / M.Pharm
Experience: 2–13 Years (QMS / IPQA / Process Validation / Cleaning Validation / AQA / AMT / AMV)
Key Responsibilities:
- Execute IPQA activities during manufacturing and packing.
- Handle deviation, CAPA, change control, and QMS documentation.
- Conduct process validation and cleaning validation activities.
- Perform line clearance and batch record review.
- Support regulatory inspections and compliance audits.
This role is critical in maintaining regulatory compliance in OSD manufacturing facilities.
Production (OSD)
Designation: Technical Associate / Sr. Technical Associate / Officer / Sr. Officer
Qualification: ITI, Diploma, B.Sc, B.Pharm
Experience: 2–9 Years (Compression / Granulation / Packing / Coating / Blister Packing)
Key Responsibilities:
- Operate compression and granulation equipment.
- Handle coating and blister packing lines.
- Maintain BMR documentation and process records.
- Ensure adherence to cGMP and safety norms.
- Coordinate with QA for in-process checks.
Engineering Services (OSD / API)
Designation: Technical Associate / Sr. Technical Associate
Qualification: ITI / Diploma (Mechanical, Electrical)
Experience: 2–9 Years (Plant Maintenance / Water System / HVAC)
Key Responsibilities:
- Preventive and breakdown maintenance of manufacturing equipment.
- Maintain HVAC systems in regulated areas.
- Manage purified water and utility systems.
- Maintain maintenance logs and compliance documentation.
Eligibility / Qualifications
Eligible educational backgrounds include:
M.Sc (Chemistry, Microbiology), B.Pharm, M.Pharm, B.Sc, ITI (Mechanical/Electrical), Diploma (Mechanical/Electrical Engineering).
Candidates must have 2–13 years of experience in regulated pharmaceutical plants with exposure to CGMP documentation.
Strong technical knowledge, regulatory awareness, and documentation skills are essential.
Location & Salary
Location: Indore (Pithampur), Madhya Pradesh
Salary: As per industry standards based on experience and designation.
Compensation will be aligned with OSD, API, injectable QC/QA, and engineering services benchmarks in regulated pharmaceutical manufacturing.
Application Process
- Register through the official link prior to attending the walk-in drive.
- Attend the interview on 22 February 2026.
- Carry updated CV, salary structure (CTC breakup), and Aadhar card.
Candidates should ensure they have relevant experience aligned to the department applying for.
Why Consider Macleods Indore?
Professionals joining this facility gain exposure to:
- USFDA-compliant pharmaceutical manufacturing
- Advanced QC analytical platforms
- Regulatory documentation and QMS systems
- Validation and qualification frameworks
- Stability studies and injectable microbiology testing
Such exposure enhances long-term career growth in quality assurance, regulatory affairs, production management, and pharmaceutical compliance careers.
Frequently Asked Questions (FAQs)
1. Who can attend this walk-in drive?
Candidates with 2–13 years of experience in QC, QA, Production, or Engineering in regulated pharma plants.
2. Is injectable QC experience mandatory for injectable roles?
Yes. Experience in sterile manufacturing and microbiology testing is preferred.
3. Are freshers eligible?
No. All positions require prior pharmaceutical experience.
4. Is registration mandatory?
Yes. Candidates must register before attending.
5. Does Macleods charge recruitment fees?
No. The company does not charge any fees for hiring.
Summary
| Company | Macleods Pharmaceuticals |
|---|---|
| Vacancies | QC (OSD/API), QC (Injectables), QA (OSD), Production (OSD), Engineering Services |
| Required Education | M.Sc (Chemistry/Microbiology), B.Pharm, M.Pharm, B.Sc, ITI, Diploma (Mechanical/Electrical) |
| Experience | 2–13 Years in regulated pharmaceutical manufacturing |