BDR Walk-in Production, QC, Warehouse

- Walk-In Interview Details
- Company Overview
- Job Role & Responsibilities
- Production – Injectables (General Injection / Overall Injection)
- Warehouse – Injectable / OSD
- Production – OSD
- OSD Packing & QMS
- Quality Control – Chemical & QMS
- ADL – Analytical Development Laboratory
- Eligibility / Qualifications
- Location & Salary
- Application Process
- Why Join BDR Pharmaceuticals?
- Frequently Asked Questions (FAQs)
- Summary
BPharm ITI Pharma Vacancies Baska Gujarat
B.Pharm, M.Pharm, BSc, ITI pharma vacancies at BDR Gujarat. Production, QC, Warehouse roles. Walk-in 21 Feb 2026.
BDR Pharmaceuticals International Pvt. Ltd. is conducting a walk-in drive for multiple positions across Production (OSD & Injectables), Warehouse, Quality Control, ADL, and QMS functions at its Gujarat manufacturing facility. Professionals with experience in regulated pharmaceutical plants, especially USFDA-approved facilities, are strongly encouraged to attend.
If you are actively searching for injectable production jobs in Gujarat, OSD technician vacancies, pharmaceutical warehouse manager openings, QC HPLC analyst roles, or ADL executive positions in a regulated pharma plant, this hiring drive offers substantial growth opportunities within a compliance-driven organization.
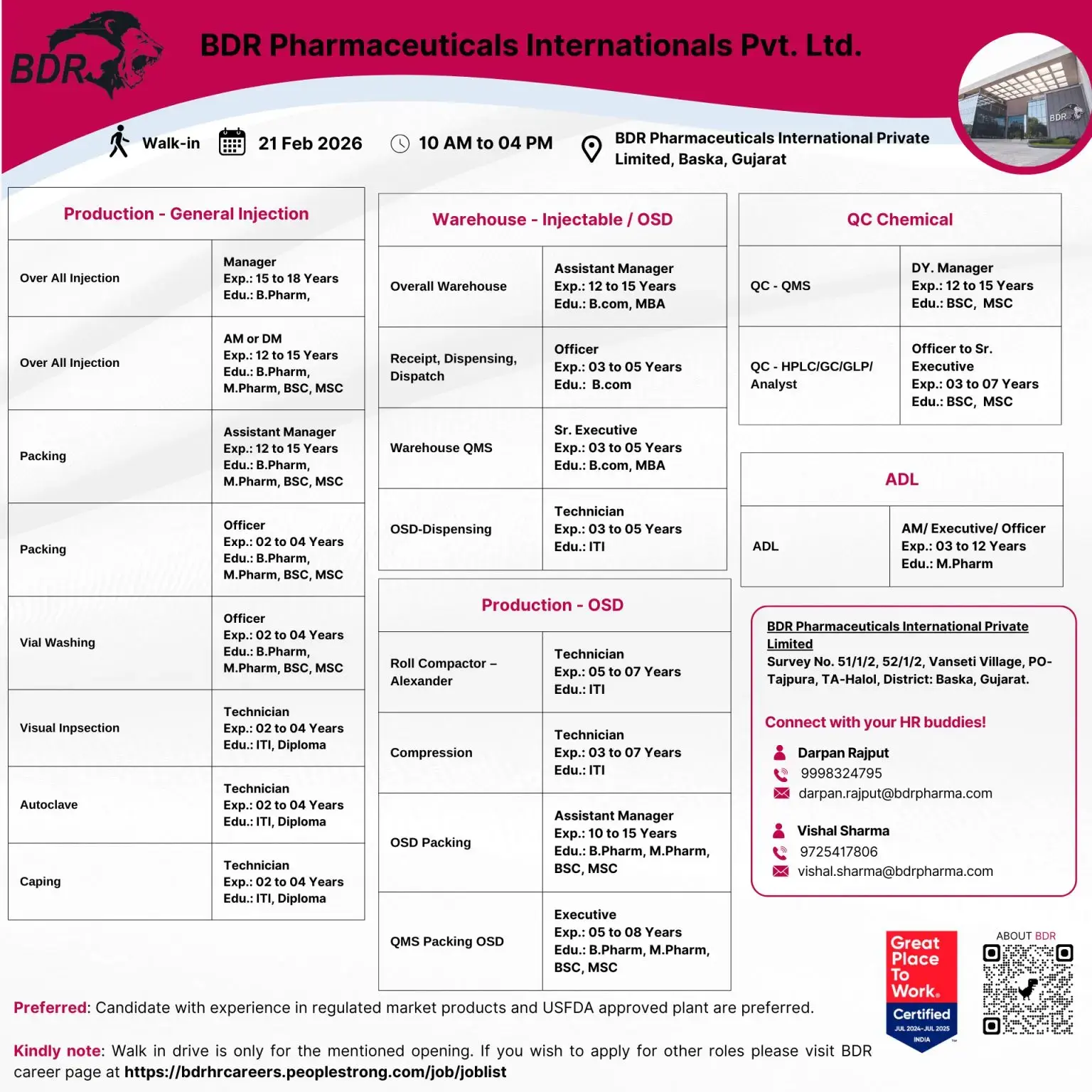
Walk-In Interview Details
Date: 21 February 2026
Time: 10:00 AM – 04:00 PM
Venue: BDR Pharmaceuticals International Pvt. Ltd.
Survey No. 51/1/2, 52/1/2, Vanseti Village, PO-Tajpura, TA-Halol, District: Baska, Gujarat
HR Contact:
- Darpan Rajput – 9998324795 – darpan.rajput@bdrpharma.com
- Vishal Sharma – 9725417806 – vishal.sharma@bdrpharma.com
Preferred: Candidates with exposure to regulated market products and USFDA-approved plants.
Company Overview
BDR Pharmaceuticals International Pvt. Ltd. is a fast-growing pharmaceutical company with strong expertise in oncology and specialty formulations. Recognized as a Great Place to Work (Certified JUL 2024 – JUL 2025), BDR operates manufacturing facilities aligned with global regulatory standards including USFDA compliance.
The company manufactures oral solid dosage forms and sterile injectable products for domestic and international markets. BDR emphasizes Quality Management Systems (QMS), GMP compliance, validation protocols, and audit readiness to meet global regulatory expectations.
Professionals joining BDR gain structured exposure to regulated manufacturing environments, deviation management systems, validation documentation, and advanced pharmaceutical production technologies.
Job Role & Responsibilities
Production – Injectables (General Injection / Overall Injection)
Designation: Manager
Experience: 15–18 Years
Qualification: B.Pharm
Responsibilities include overall injectable production management, vial washing, visual inspection, autoclave operations, capping, and packing supervision while ensuring sterile compliance and regulatory documentation.
Warehouse – Injectable / OSD
Designations: AM / DM / Assistant Manager / Officer
Experience: 2–15 Years (Role Dependent)
Qualification: B.Pharm, M.Pharm, B.Sc, M.Sc
Key Responsibilities:
- Overall warehouse operations for injectable and OSD units.
- Receipt, dispensing, and dispatch management.
- Warehouse QMS documentation and compliance.
- Inventory control and regulated documentation practices.
Production – OSD
Designations: Technician / Executive / Assistant Manager
Experience: 3–15 Years
Qualification: ITI, B.Pharm, M.Pharm, B.Sc, M.Sc
Responsibilities:
- Operate roll compactor (Alexander), compression machines.
- Execute granulation and packing activities.
- Maintain BMR documentation and GMP compliance.
- Coordinate with QA and QC for batch release.
OSD Packing & QMS
Designations: Assistant Manager / Sr. Executive / Officer / Technician
Experience: 3–15 Years
Qualification: B.Com, MBA, ITI
Responsibilities:
- Handle OSD packing operations.
- Manage packing QMS documentation and deviation handling.
- Supervise serialization, labeling, and batch compliance activities.
Quality Control – Chemical & QMS
Designations: Dy. Manager / Officer / Sr. Executive
Experience: 3–15 Years
Qualification: B.Sc, M.Sc
Key Responsibilities:
- Perform HPLC, GC, GLP-based analytical testing.
- Manage laboratory calibration and documentation.
- Conduct RM, PM, and finished product analysis.
- Handle QC-QMS systems and regulatory audit support.
ADL – Analytical Development Laboratory
Designations: AM / Executive / Officer
Experience: 3–12 Years
Qualification: M.Pharm
Responsibilities:
- Method development and validation.
- Analytical transfer documentation.
- Support regulatory submission data preparation.
- Maintain compliance with GLP and data integrity standards.
Eligibility / Qualifications
Relevant Educational Background Includes:
ITI (Technical Trades), B.Pharm, M.Pharm, B.Sc (Chemistry), M.Sc (Chemistry), B.Com, MBA.
Candidates must have prior pharmaceutical manufacturing exposure. Experience in regulated or USFDA-approved facilities is preferred.
Location & Salary
Location: Halol / Baska District, Gujarat
Salary: Competitive and aligned with industry standards depending on experience and regulatory exposure.
Gujarat remains a major pharmaceutical manufacturing hub, offering long-term stability and career growth in API, OSD, and sterile injectable domains.
Application Process
Candidates should attend the walk-in with:
- Updated Resume
- Educational Certificates
- Experience Letters
- Last 3 Months Payslips
- ID Proof
For other roles, candidates may apply via the official BDR career page.
Why Join BDR Pharmaceuticals?
- Exposure to regulated pharmaceutical manufacturing
- Work in USFDA-compliant production systems
- Structured QMS and validation environment
- Career progression in oncology and specialty manufacturing
- Recognized Great Place to Work culture
Hands-on experience in regulated injectable and OSD manufacturing significantly enhances long-term career prospects in Quality Assurance, Regulatory Affairs, Validation, and Senior Production Leadership roles.
Frequently Asked Questions (FAQs)
1. Is USFDA plant experience mandatory?
Preferred but not mandatory for all roles.
2. Are freshers eligible?
Most roles require experience; check specific eligibility.
3. Which departments are hiring?
Production (Injectable & OSD), Warehouse, QC, ADL, QMS.
4. Can candidates apply online?
Yes, via official BDR career portal for roles beyond walk-in listings.
5. What qualifications are accepted?
ITI, B.Pharm, M.Pharm, B.Sc, M.Sc, B.Com, MBA.
Summary
| Company | BDR Pharmaceuticals International Pvt. Ltd. |
|---|---|
| Vacancies | Production (Injectable & OSD), Warehouse Manager/Officer, QC Chemical Analyst, ADL Executive, QMS Roles |
| Required Education | ITI, B.Pharm, M.Pharm, B.Sc, M.Sc, B.Com, MBA |
| Experience | 2–18 Years (Role Dependent) |
To apply for this job email your details to vishal.sharma@bdrpharma.com


