BioMatrix walk-in API Production, QC, R&D, EHS

- Walk-In Interview Details
- Company Overview
- Job Role & Responsibilities
- Production – API Manufacturing
- Process Engineer – API
- Quality Control – API
- Research & Development – API
- EHS – Environment, Health & Safety
- Eligibility / Qualifications
- Location & Salary
- Application Process
- Why Join BioMatrix API Operations?
- Frequently Asked Questions (FAQs)
- Summary
API Officer & Executive Vacancies Vadodara
API Production, QC, R&D, EHS vacancies at BioMatrix Vadodara. 2–10 years experience. Walk-in 28 Feb 2026 Ankleshwar.
BioMatrix Healthcare is conducting a walk-in interview for experienced professionals in API manufacturing, Quality Control, Research & Development, QMS, Process Engineering, and EHS functions. The hiring drive will be held in Ankleshwar for positions at its API manufacturing facility near Vadodara, Gujarat. Candidates with prior exposure to pharmaceutical API plants and regulated manufacturing environments are encouraged to attend.
This opportunity is suitable for professionals seeking API production jobs in Gujarat, pharmaceutical quality control executive roles, API process engineer vacancies, R&D synthesis openings, GLP laboratory careers, and EHS officer jobs in pharmaceutical manufacturing.
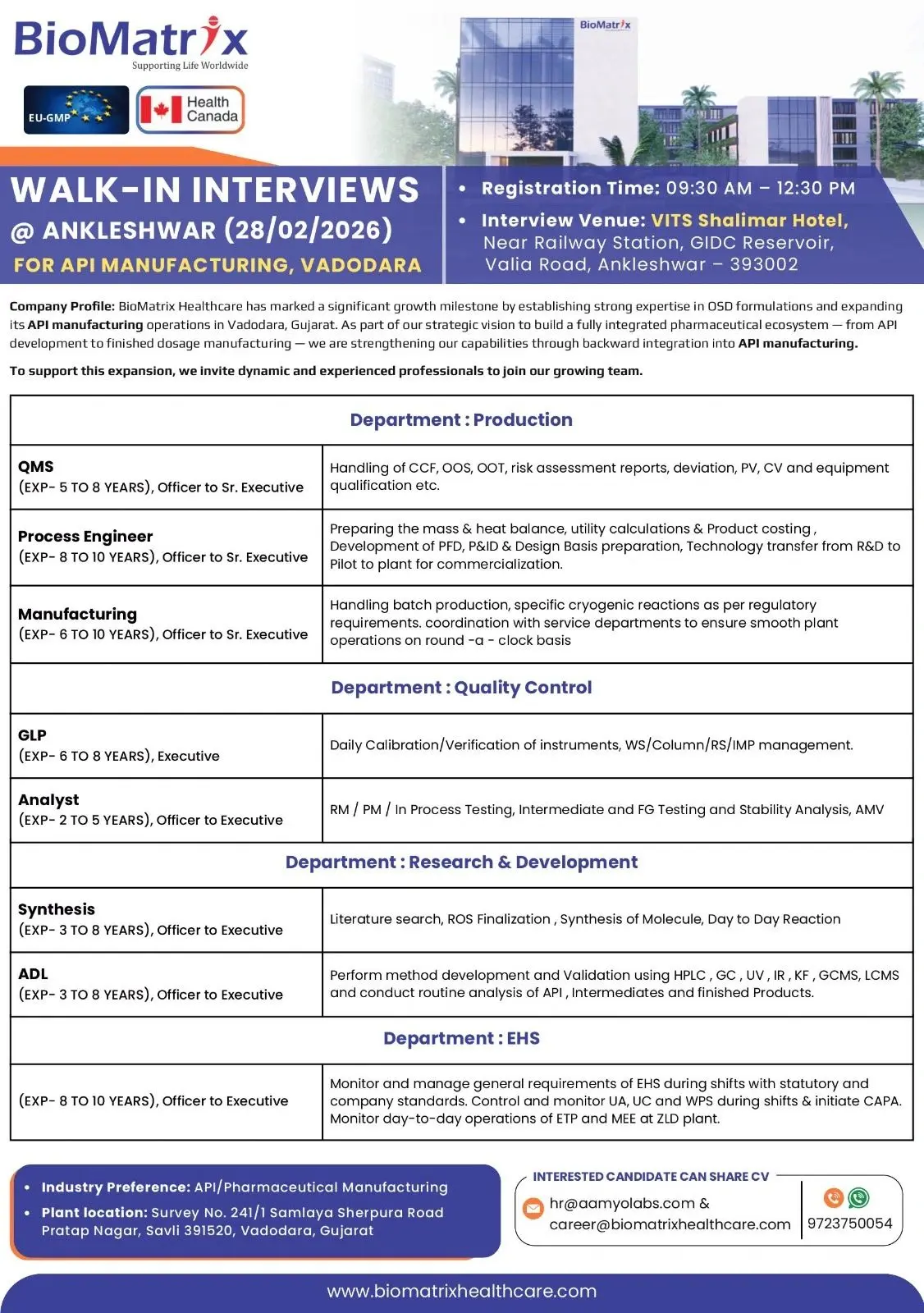
Walk-In Interview Details
Date: 28 February 2026
Registration Time: 09:30 AM – 12:30 PM
Venue: VITS Shalimar Hotel, Near Railway Station, GIDC Reservoir, Valia Road, Ankleshwar – 393002
Job Location (API Plant): Survey No. 241/1, Samlaya Sherpura Road, Pratap Nagar, Savli, Vadodara, Gujarat – 391520
Interested candidates may also share their CV at:
hr@aamyolabs.com
career@biomatrixhealthcare.com
Contact: 9723750054
Company Overview
BioMatrix Healthcare has expanded its pharmaceutical footprint by strengthening backward integration into Active Pharmaceutical Ingredient (API) manufacturing. With a growing portfolio in regulated formulations and API development, the company is building a fully integrated pharmaceutical ecosystem — from research and development to finished dosage commercialization.
The Vadodara API site focuses on compliant manufacturing systems aligned with GMP, GLP, data integrity, validation, and risk-based quality management. The organization emphasizes regulatory documentation, process validation, deviation control, and audit readiness to meet domestic and global regulatory expectations.
Professionals joining BioMatrix gain hands-on exposure to large-scale API synthesis, quality systems, validation programs, process engineering scale-up, and environmental compliance management.
Job Role & Responsibilities
Production – API Manufacturing
Designation: Officer to Sr. Executive
Experience: 6–10 Years (Manufacturing) / 5–8 Years (QMS)
Manufacturing Responsibilities
- Handle batch production activities in API manufacturing.
- Execute cryogenic and controlled reactions as per regulatory requirements.
- Coordinate with utility and service departments for uninterrupted plant operations.
- Ensure compliance with batch manufacturing records and SOPs.
- Monitor process parameters for quality and yield optimization.
QMS Responsibilities (Production QMS)
- Handle CCF, OOL, OOT investigations.
- Conduct deviation management and risk assessment reports.
- Manage Process Validation (PV) and Cleaning Validation (CV).
- Support equipment qualification documentation.
- Maintain compliance with pharmaceutical quality management systems.
Process Engineer – API
Designation: Officer to Sr. Executive
Experience: 8–10 Years
Key Responsibilities:
- Prepare mass and heat balance calculations.
- Conduct utility calculations and product costing analysis.
- Develop PFD, P&ID, and Design Basis documentation.
- Support technology transfer from R&D scale to pilot and commercial plant scale.
- Optimize process parameters for commercial manufacturing.
This role is critical for scale-up, process optimization, and commercialization of API molecules.
Quality Control – API
GLP Executive
Experience: 6–8 Years
- Daily calibration and verification of laboratory instruments.
- Manage working standards (WS), reference standards (RS), columns, and impurity profiles.
- Ensure GLP compliance and documentation integrity.
Analyst – Officer to Executive
Experience: 2–5 Years
- Conduct RM, PM, in-process, intermediate, and finished product testing.
- Perform stability analysis and AMV activities.
- Support method validation and documentation.
Exposure to regulatory audit compliance and laboratory data integrity is essential.
Research & Development – API
Synthesis (Officer to Executive)
Experience: 3–8 Years
- Perform literature search and route of synthesis (ROS) finalization.
- Execute day-to-day chemical reactions and process optimization.
- Develop scalable synthetic pathways.
- Maintain R&D documentation aligned with regulatory expectations.
ADL – Analytical Development Laboratory
Experience: 3–8 Years
- Perform analytical method development and validation.
- Operate HPLC, GC, UV, IR, KF, GCMS, LCMS instruments.
- Conduct routine analysis of API, intermediates, and finished products.
- Support regulatory documentation for method transfer.
EHS – Environment, Health & Safety
Designation: Officer to Executive
Experience: 8–10 Years
Key Responsibilities:
- Monitor statutory EHS compliance during shifts.
- Supervise UA, UC, and WPS controls.
- Implement CAPA for safety deviations.
- Monitor ETP and MEE operations at plant.
- Ensure safe handling of hazardous chemicals and waste management.
EHS is critical in API plants due to chemical reactions, solvents, and waste management requirements.
Eligibility / Qualifications
Relevant Educational Background Includes:
B.Sc (Chemistry), M.Sc (Chemistry), B.Pharm, M.Pharm, B.Tech (Chemical), M.Tech (Chemical), Diploma (Chemical Engineering).
Candidates must have prior experience in API or pharmaceutical manufacturing plants.
Industry Preference: API / Pharmaceutical Manufacturing Plant Exposure.
Location & Salary
Plant Location: Savli, Vadodara, Gujarat
Interview Location: Ankleshwar
Salary: Competitive and aligned with industry standards based on experience and regulatory exposure.
Gujarat remains a leading pharmaceutical API hub in India, offering long-term career growth in manufacturing, validation, R&D, and compliance.
Application Process
Walk-in candidates should carry:
- Updated Resume
- Experience Certificates
- Last 3 Months Payslips
- Educational Certificates
- Government ID Proof
Candidates unable to attend may email their CV to:
hr@aamyolabs.com
career@biomatrixhealthcare.com
Why Join BioMatrix API Operations?
API manufacturing is one of the most technically demanding segments of the pharmaceutical industry. Professionals here gain expertise in:
- Regulatory compliance & validation systems
- Chemical process scale-up
- GLP & GMP laboratory management
- Risk assessment & deviation handling
- EHS and statutory compliance systems
Such exposure significantly enhances long-term earning potential in regulated pharmaceutical manufacturing careers.
Frequently Asked Questions (FAQs)
1. Is API experience mandatory?
Yes. Preference is given to candidates from API or pharmaceutical manufacturing plants.
2. Are freshers eligible?
No. All roles require relevant industry experience.
3. What documents are required for walk-in?
Resume, payslips, certificates, and ID proof.
4. Is this for formulation manufacturing?
The focus is on API manufacturing operations.
5. Can candidates email their resume instead of walk-in?
Yes, resumes can be shared on the provided email IDs.
Summary
| Company | BioMatrix Healthcare |
|---|---|
| Vacancies | Production (API), QMS Officer, Process Engineer, QC GLP Executive, QC Analyst, R&D Synthesis Officer, ADL Executive, EHS Officer |
| Required Education | B.Sc (Chemistry), M.Sc (Chemistry), B.Pharm, M.Pharm, B.Tech (Chemical), M.Tech (Chemical), Diploma (Chemical Engineering) |
| Experience | 2–10 Years (Role Dependent) |
To apply for this job email your details to hr@aamyolabs.com

