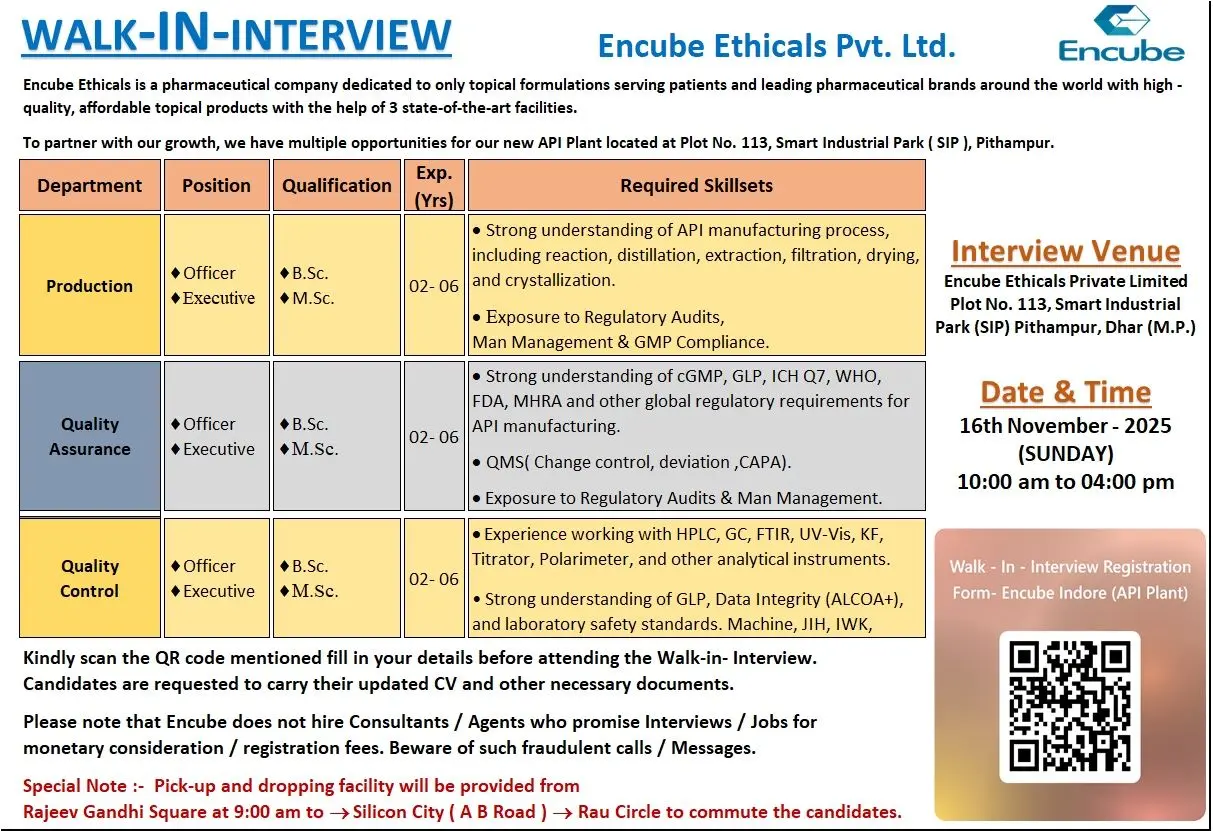
Encube Ethicals Walk-in API Production, QA, and QC

- Company Overview
- Job Role & Responsibilities
- Production – Officer / Executive
- Quality Assurance – Officer / Executive
- Quality Control – Officer / Executive
- Eligibility / Qualifications
- Location & Salary
- Application Process
- FAQs
- Summary Table
B.Sc/M.Sc API Production & QC Roles at Encube Pithampur
Encube Ethicals hiring for API Production, QA, and QC at Pithampur. Multiple B.Sc/M.Sc vacancies with 2–6 years experience.
Encube Ethicals is hiring experienced professionals for its new API manufacturing plant at Pithampur. This expansion opens strong career opportunities for candidates skilled in API production processes, analytical testing, regulatory compliance, and quality management. The walk-in interview is scheduled for 16 November 2025, making this an ideal opportunity for candidates who want to join a fast-growing pharmaceutical company with a strong global footprint.
Company Overview
Encube Ethicals Pvt. Ltd. is a specialized pharmaceutical organization focused exclusively on topical formulations. The company serves global markets with high-quality, affordable products manufactured across three state-of-the-art facilities. With the launch of its new API plant in Smart Industrial Park (SIP), Pithampur, Encube is strengthening its backward integration, enhancing quality control, and expanding its global supply capabilities.
Encube maintains strict compliance aligned with cGMP, ICH Q7, WHO guidelines, FDA, MHRA expectations, and other international regulatory standards. This environment offers strong learning and growth for candidates aiming to build expertise in API technology and regulatory-driven operations.
Job Role & Responsibilities
Encube has multiple openings in Production, Quality Assurance, and Quality Control for its API facility.
Production – Officer / Executive
Qualifications: B.Sc (Officer), M.Sc (Executive)
Experience: 2–6 years
Key responsibilities:
- Manage API manufacturing steps such as reaction, distillation, extraction, crystallization, filtration, and drying.
- Monitor batch process parameters and ensure adherence to cGMP.
- Support scale-up activities, equipment handling, and process optimization.
- Ensure documentation accuracy and compliance during batch operations.
- Coordinate with QA, QC, and engineering for smooth production workflow.
Quality Assurance – Officer / Executive
Qualifications: B.Sc, M.Sc
Experience: 2–6 years
Key responsibilities:
- Manage QA activities including change control, deviation, CAPA, and QMS oversight.
- Review batch manufacturing records, laboratory documents, and SOP compliance.
- Support internal and external audits, regulatory inspections, and documentation control.
- Ensure adherence to ICH Q7, WHO, FDA, MHRA, and global regulatory requirements.
- Coordinate with production teams for continuous quality improvement.
Quality Control – Officer / Executive
Qualifications: B.Sc (Officer), M.Sc (Executive)
Experience: 2–6 years
Key responsibilities:
- Perform analytical testing for raw materials, intermediates, and API using HPLC, GC, FTIR, UV-Vis, KF, Titrator, and Polarimeter.
- Ensure GLP compliance, data integrity (ALCOA+), and laboratory safety.
- Support method validation, stability studies, and impurity profiling.
- Troubleshoot analytical instruments and maintain calibration schedules.
- Prepare COAs, analytical reports, and regulatory-aligned documentation.
Eligibility / Qualifications
- Required Education: B.Sc, M.Sc in Chemistry or related fields.
- Relevant Courses: B.Sc Chemistry, M.Sc Organic Chemistry, Analytical Chemistry, Pharmaceutical Chemistry, Industrial Chemistry.
- Experience working in API plants with exposure to GMP documentation.
- Strong understanding of cGMP, GLP, QMS, ICH Q7, and regulatory audits.
- Hands-on experience with major analytical instruments for QC roles.
- Good communication skills and ability to work in a regulated environment.
Location & Salary
Walk-in Venue: Encube Ethicals Private Limited, Plot No. 113, Smart Industrial Park (SIP), Pithampur, Dhar (M.P.)
Interview Date & Time: 16 November 2025 (Sunday), 10:00 AM – 4:00 PM
Transport Facility: Pick-up and drop from Rajeev Gandhi Square to Silicon City (AB Road) → Rau Circle.
Salary will be based on experience, technical skill, and regulatory exposure. API, QC, and QA professionals typically command higher salary brackets due to specialized expertise.
Application Process
Before attending the walk-in, candidates must complete the registration form:
Walk-In Registration Form: Scan the QR code mentioned in the official notification.
Bring the following documents:
- Updated CV
- Educational certificates
- Experience letters
- ID proof and passport-size photographs
Important Note: Encube does not hire consultants or agents. Do not make any payment to anyone for interview or job placement.
FAQs
1. Who can apply for these roles?
Candidates with 2–6 years of relevant API Production, QA, or QC experience and B.Sc/M.Sc qualifications.
2. Are freshers eligible?
No. All positions require a minimum of 2 years of experience.
3. What analytical skills are required for QC roles?
Experience with HPLC, GC, FTIR, UV-Vis, KF, Titrator, and Polarimeter.
4. Is regulatory audit exposure mandatory?
Preferred for all roles. Candidates with FDA/MHRA exposure will be prioritized.
5. What should candidates bring for the interview?
CV, certificates, experience documents, and identification proof.
Summary Table
| Company | Encube Ethicals Pvt. Ltd. |
|---|---|
| Vacancies | Production, QA, QC (Officers & Executives) |
| Required Education | B.Sc, M.Sc, Chemistry, Analytical Chemistry, Organic Chemistry |
| Experience | 2–6 years in API Production, Quality Assurance, or Quality Control |