Eris Hiring QC, QA, and Engineering

- Company Overview
- Job Role & Responsibilities
- Quality Control (QC)
- OSD – Granulation
- Production – Operator
- Quality Assurance (QA) – IPQA
- Engineering – Instrumentation
- Eligibility / Qualifications
- Location & Salary
- Application Process
- Summary Table
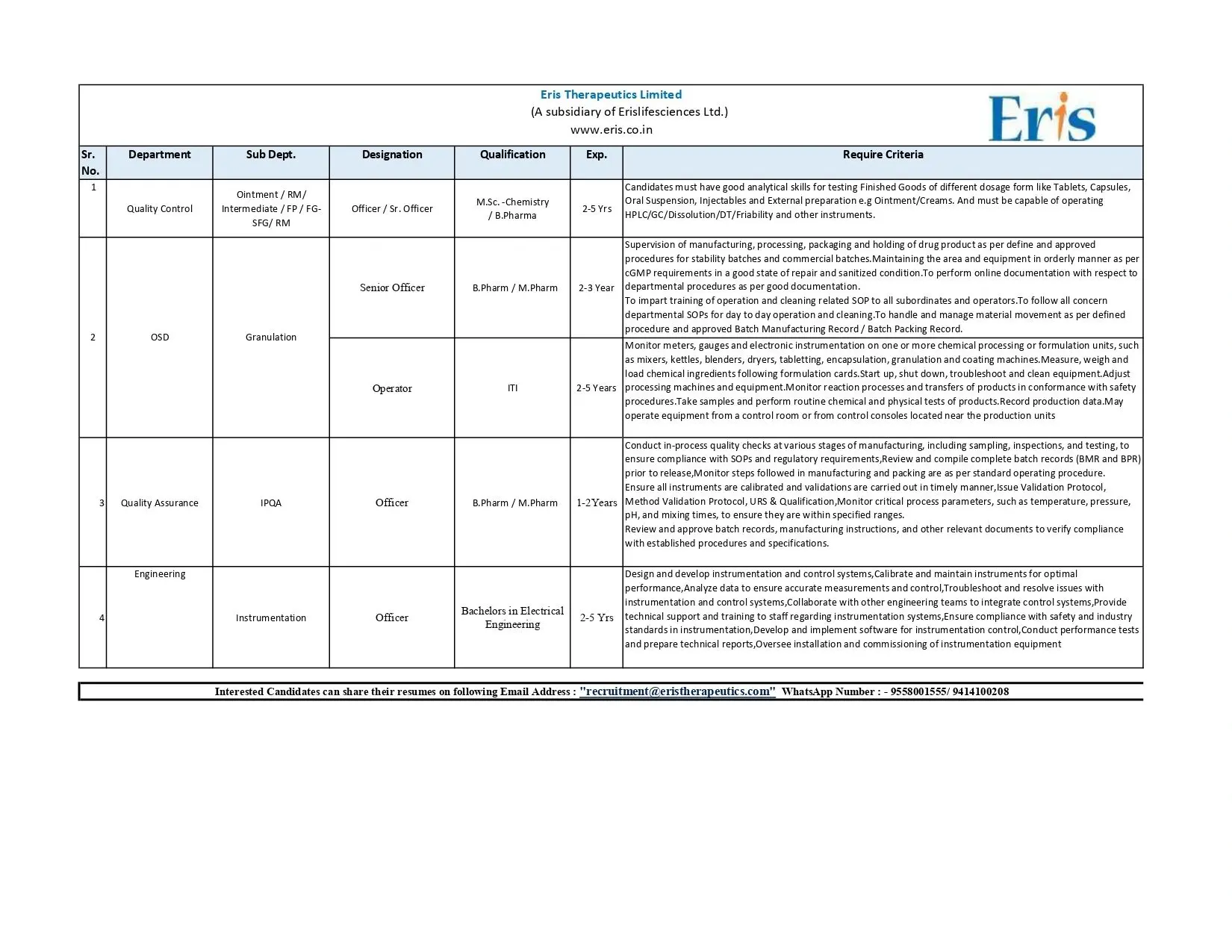
QC, QA, Engineering Openings for B.Pharm/M.Sc – Ahmedabad
Eris Therapeutics hiring QC, QA, and Engineering professionals. Multiple vacancies for B.Pharm, M.Pharm, M.Sc. Location: Ahmedabad.
Eris Therapeutics Limited is expanding its quality and manufacturing teams and is seeking skilled professionals across QC, QA, OSD production, and engineering. These openings offer a strong career path for candidates with hands-on experience in analytical testing, OSD manufacturing, documentation, and instrumentation.
Company Overview
Eris Therapeutics Limited, a subsidiary of Eris Lifesciences Ltd., is a well-recognized pharmaceutical organization known for its high-quality formulations and advanced manufacturing standards. With a strong and compliant facility, the company focuses on producing a wide range of dosage forms, including tablets, capsules, oral suspensions, injectables, ointments, and creams. Eris continues to strengthen its workforce to meet growing market and regulatory demands.
Job Role & Responsibilities
Quality Control (QC)
Designation: Officer / Senior Officer
Areas: Ointment, RM, Intermediate, FP, FG-SFG
Key Responsibilities:
- Perform analytical testing on multiple dosage forms including tablets, capsules, oral suspensions, injectables, ointments, and creams.
- Operate laboratory instruments such as HPLC, GC, Dissolution, DT, and Friability testers.
- Execute stability and routine sample analysis.
- Maintain accurate documentation in line with cGMP requirements.
OSD – Granulation
Designation: Senior Officer
Responsibilities:
- Manage granulation activities for OSD manufacturing.
- Supervise material handling, processing, and batch operations.
- Maintain manufacturing area cleanliness as per SOP.
- Perform online documentation and ensure compliance during commercial and stability batches.
Production – Operator
Responsibilities:
- Operate and maintain formulation equipment such as mixers, blenders, dryers, compression, encapsulation, granulation, and coating machines.
- Load and measure raw materials following formulation instructions.
- Conduct routine quality checks and record production parameters.
- Perform equipment cleaning, troubleshooting, and adjustments.
Quality Assurance (QA) – IPQA
Designation: Officer
Responsibilities:
- Conduct in-process checks across manufacturing stages.
- Review BMR and BPR prior to batch release.
- Ensure compliance with SOPs and regulatory standards.
- Monitor and document critical parameters such as pH, temperature, mixing times.
- Issue validation and qualification protocols whenever required.
Engineering – Instrumentation
Designation: Officer
Responsibilities:
- Maintain and calibrate instrumentation and control systems.
- Analyze performance data and ensure accurate system outputs.
- Troubleshoot equipment and collaborate with engineering teams.
- Support installation, commissioning, and documentation activities.
- Conduct performance tests and prepare technical reports.
Eligibility / Qualifications
- QC: M.Sc Chemistry, B.Pharm with 2–5 years experience.
- OSD Production: B.Pharm/M.Pharm with 2–3 years experience.
- Operator: Experience of 2–5 years in chemical or formulation units.
- QA – IPQA: B.Pharm/M.Pharm with 1–2 years experience.
- Instrumentation: Bachelor’s degree in Electrical Engineering with 2–5 years experience.
Location & Salary
Location: Ahmedabad (Gujarat)
Salary: As per industry standards and based on experience.
Application Process
Interested candidates can send their updated CV to:
recruitment@eristherapeutics.com
WhatsApp: 9558001555 / 9414100208
Website: www.eris.co.in
Summary Table
| Company | Eris Therapeutics Limited |
|---|---|
| Vacancies | QC, QA, OSD Production, Operator, Instrumentation |
| Required Education | B.Pharm, M.Pharm, M.Sc Chemistry, Electrical Engineering |
| Experience | 1–5 Years (role-based) |
You must sign in to apply for this position.


