Eris Therapeutics Hiring QC, Manufacturing, IPQA

- M.Pharm/B.Pharm QC & Manufacturing Roles | Eris (Mandate)
- Company Overview
- Job Role & Responsibilities
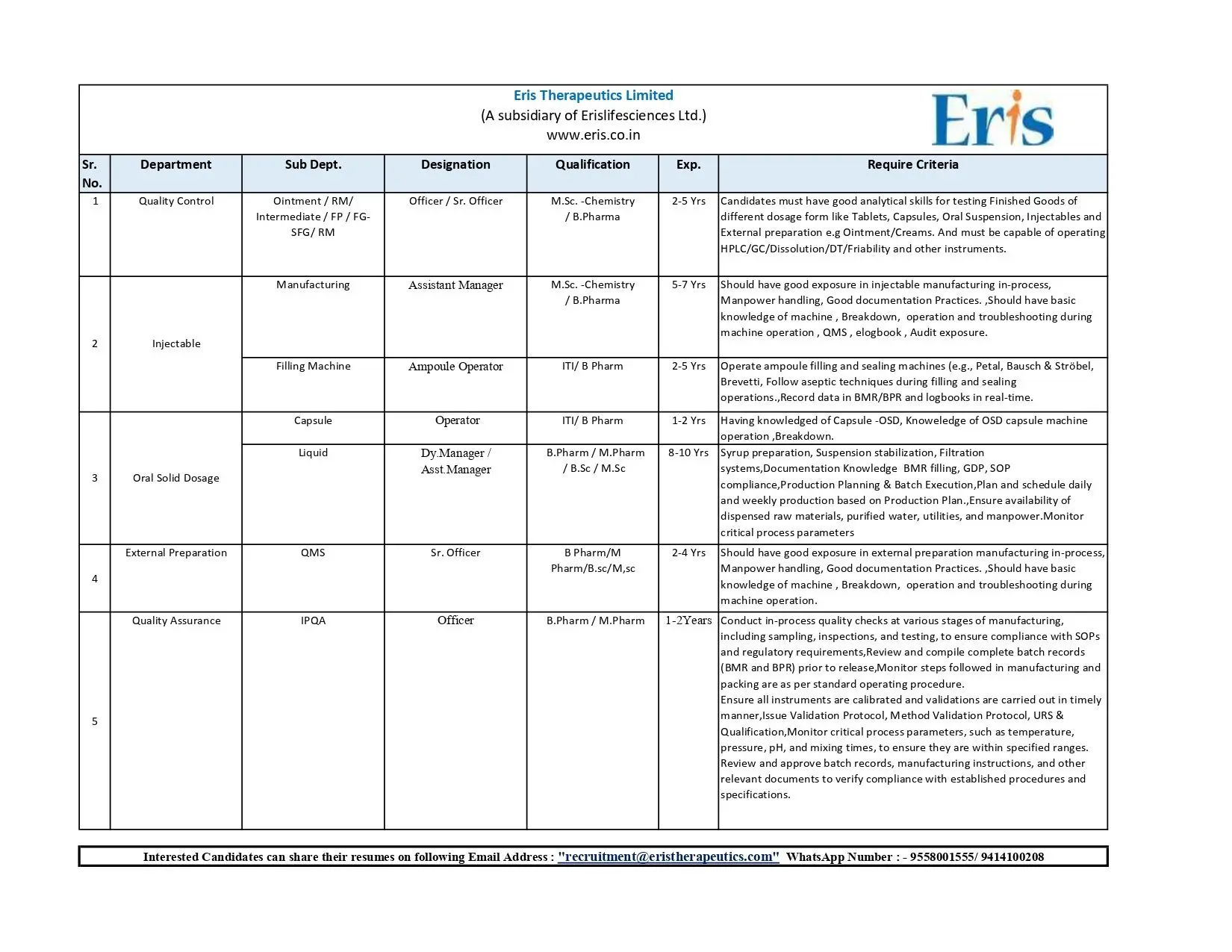
- Quality Control – Officer / Sr. Officer
- Manufacturing – Assistant Manager / Dy. Manager / Asst. Manager / Operator
- IPQA – Officer
- QMS / External Preparations – Sr. Officer
- Eligibility / Qualifications
- Location & Salary
- Why this role matters
- Application Process
- Selection Process & Tips
- FAQs
- Summary Table
M.Pharm/B.Pharm QC & Manufacturing Roles | Eris (Mandate)
Eris Therapeutics hiring B.Pharm/M.Sc/M.Pharm for QC, Manufacturing, IPQA roles. Multiple vacancies—2–10 years experience preferred.
Eris Therapeutics is recruiting experienced pharmaceutical professionals across Quality Control, Manufacturing, IPQA and QMS roles. These openings suit candidates with hands‑on analytical skills (HPLC/GC/Dissolution), injectable and OSD manufacturing experience, and a strong grasp of cGMP and regulatory expectations. If you are seeking a role that contributes directly to medicine quality and patient safety, this hiring drive offers meaningful, career-building responsibilities.
Company Overview
Eris Therapeutics Limited is part of Erislifesciences and operates with a clear focus on high-quality formulations across multiple dosage forms. The company emphasizes robust quality systems, regulatory compliance, and manufacturing excellence. Eris’ teams work on tablets, capsules, syrups, injectables, and external preparations—giving you exposure to diverse pharmaceutical technologies and compliance practices such as GLP, GMP, and data integrity (ALCOA+).
Job Role & Responsibilities
Eris has multiple openings across Quality Control, Manufacturing, IPQA (In-Process Quality Assurance), and QMS functions. Below are role-specific responsibilities and expectations.
Quality Control – Officer / Sr. Officer
Qualification: M.Sc (Chemistry) / B.Pharm
Experience: 2–5 years
Responsibilities:
- Perform routine and release testing of Finished Goods across Tablets, Capsules, Oral Suspensions, Injectables, and Ointments/Creams.
- Operate analytical instruments: HPLC, GC, Dissolution, Disintegration, Friability, UV-Vis, Karl Fischer, Titrators, Polarimeter.
- Execute method validation, system suitability, and stability study sampling.
- Investigate OOS/OOT events, write technical reports, and implement corrective actions.
- Ensure GLP compliance, data integrity (ALCOA+), and accurate COA preparation for regulatory submissions.
Manufacturing – Assistant Manager / Dy. Manager / Asst. Manager / Operator
Qualification: M.Sc Chemistry / B.Pharm / ITI (where specified)
Experience Range: 2 – 10 years depending on designation
Responsibilities:
- Oversee injectable manufacturing lines: filling, sealing (ampoules), sterilization, and aseptic handling.
- Manage OSD production: capsule & tablet operations, syrup and suspension preparation, filtration, and batch execution.
- Plan daily/weekly production schedules and ensure availability of materials, utilities, and manpower.
- Maintain BMR/BPR accuracy and enforce GMP practices on the shop floor.
- Troubleshoot equipment breakdowns, support preventive maintenance, and coordinate with engineering for uptime optimization.
IPQA – Officer
Qualification: B.Pharm / M.Pharm
Experience: 1–2 years
Responsibilities:
- Conduct in-process quality checks, sampling, and inspections across manufacturing stages.
- Review and compile batch records (BMR/BPR) prior to release.
- Monitor compliance to SOPs, critical process parameters, and calibration schedules.
- Support validation, issuance of protocols (Validation, Method Validation, URS), and facilitate deviation investigations.
QMS / External Preparations – Sr. Officer
Qualification: B.Pharm / M.Pharm / B.Sc / M.Sc
Experience: 2–4 years
Responsibilities:
- Manage QMS activities: change controls, deviations, CAPA, document control, and training records.
- Oversee manufacturing of external preparations (ointments, creams), ensuring in-process checks and personnel management.
- Support internal and external audits; prepare responses and corrective action plans.
Eligibility / Qualifications
Required qualifications and relevant courses (comma-separated): M.Sc Chemistry, B.Pharm, M.Pharm, B.Sc Chemistry, D.Pharm, Diploma in Pharmacy, Industrial Training in Pharma Technology.
Essential experience and skills:
- 2–10 years in pharmaceutical manufacturing, QC analysis, or IPQA depending on role.
- Proven hands-on experience with HPLC/GC/Dissolution and other analytical instrumentation.
- Injectable manufacturing exposure preferred for relevant positions (ampoule filling, sterile handling).
- Strong understanding of cGMP, GLP, ICH guidelines, and data integrity (ALCOA+).
- Experience with QMS tools, CAPA, deviation handling, and audit responses.
- Good documentation practices, technical report writing, and teamwork.
Location & Salary
Location: Positions are with Eris Therapeutics (refer to company communication for specific plant location).
Salary: Competitive and commensurate with experience, role, and technical expertise. Exact compensation will be shared during the selection process.
Why this role matters
Working at Eris places you at the intersection of manufacturing and patient safety. Your work—whether validating an HPLC method, investigating an OOS, or ensuring sterile ampoule fills—directly impacts product quality and compliance. That contributes to safer medicines and strengthens the healthcare supply chain.
Application Process
Interested candidates should share their CV and contact details by email or WhatsApp:
- Email: recruitment@eristherapeutics.com
- WhatsApp: 9558001555 / 9414100208
When applying, include:
- Current resume with academic percentages and experience details.
- List of instruments you have operated and validation/method development exposure.
- Contactable references and notice period.
Selection Process & Tips
- Shortlisted candidates will be contacted for technical interview followed by HR discussion.
- Be prepared to discuss: method validation projects, OOS investigations you handled, sterile operations (if applicable), and corrective actions you implemented.
- Bring scanned copies of degree certificates, experience letters, and one passport-size photo when requested.
FAQs
Q1. Are freshers eligible to apply?
A1. These positions require 2+ years experience for most roles. Entry-level opportunities are limited.
Q2. Is injectable experience mandatory?
A2. Injectable experience is mandatory for roles explicitly mentioning ampoule filling and injectable manufacturing; for other roles it’s preferred.
Q3. What analytical skills are essential?
A3. HPLC and GC operation, dissolution testing, and other wet lab techniques. Method validation experience is a plus.
Q4. Does Eris conduct regulatory audits?
A4. Yes—candidates with prior exposure to FDA/MHRA or other regulatory inspections will be prioritized.
Q5. How long before I hear back?
A5. Typical timelines vary; shortlisted applicants will be contacted within 1–3 weeks depending on volume.
Summary Table
| Company | Eris Therapeutics Limited |
|---|---|
| Vacancies | QC Officers/Sr Officers, Manufacturing (Assistant Manager/Dy Manager/Operators), IPQA Officer, QMS/Sr Officer |
| Required Education | M.Sc (Chemistry), B.Pharm, M.Pharm, B.Sc (Chemistry), D.Pharm, Diploma in Pharmacy |
| Experience | 1–10 years depending on role; 2–5 years typical for QC/Manufacturing; injectable and regulatory audit exposure preferred |
To apply for this job email your details to recruitment@eristherapeutics.com


