Ipca Walk-in QA, QC, Production & Packing

- Company Overview
- Job Role & Responsibilities
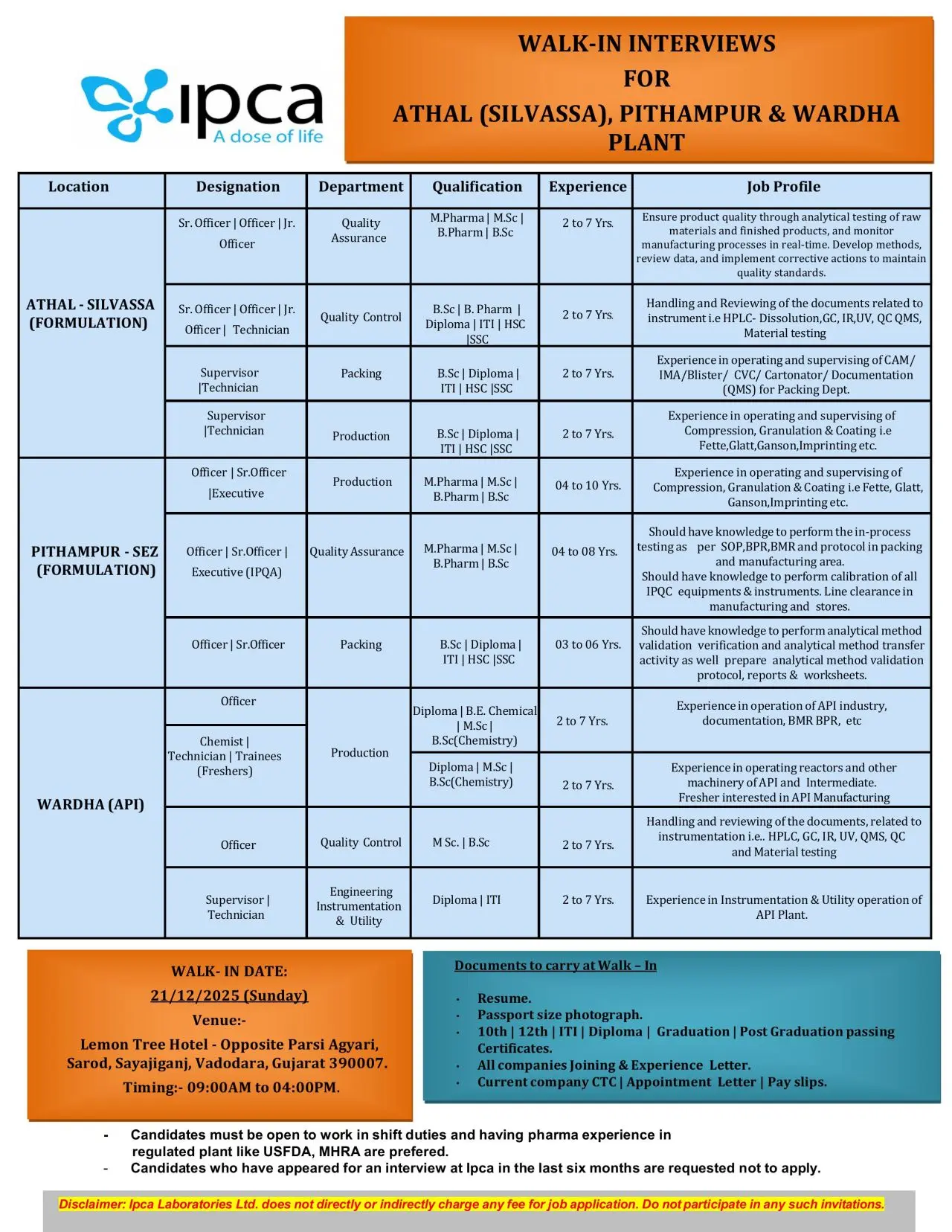
- Athal – Silvassa (Formulation)
- Quality Assurance
- Quality Control
- Packing Department
- Production Department
- Pithampur – SEZ (Formulation)
- Quality Assurance (IPQA)
- Quality Control
- Wardha (API)
- Production
- Trainees (Freshers)
- Quality Control
- Engineering – Instrumentation & Utility
- Eligibility / Qualifications
- Location & Salary
- Application Process
- FAQs
- Summary Table
Ipca Walk-in for QA, QC, Production & Packing Roles | BSc MSc Diploma | Vadodara
Ipca hiring for QA, QC, Production, Packing, Engineering across Athal, Silvassa, Pithampur & Wardha. Walk-in on 21 Dec 2025 in Vadodara.
Ipca Laboratories is conducting a large-scale walk-in interview drive for multiple departments across its formulation and API manufacturing units located in Athal (Silvassa), Pithampur SEZ, and Wardha. This recruitment drive is open to candidates with qualifications ranging from ITI, Diploma, HSC, B.Sc, B.Pharm, M.Pharm, and M.Sc, with roles suited for freshers as well as experienced professionals. Candidates with experience in regulated plants such as USFDA and MHRA are preferred.
Company Overview
Ipca Laboratories Ltd. is a trusted global pharmaceutical company known for its strong manufacturing capabilities, regulatory compliance, and high-quality formulations and APIs. With multiple plants across India and a wide global footprint, Ipca plays a significant role in producing essential medicines, APIs, and intermediates. Working with Ipca offers exposure to world-class manufacturing systems, advanced equipment, and a structured quality environment.
Job Role & Responsibilities
Ipca is hiring for several departments across its manufacturing units. The roles involve hands-on responsibilities aligned with quality systems, production operations, instrumentation, and packaging.
Athal – Silvassa (Formulation)
Quality Assurance
Designation: Sr. Officer / Officer
Qualification: M.Sc, M.Pharm, B.Pharm
Experience: 2–7 years
Responsibilities:
- Performing analytical testing of raw materials and finished products
- Monitoring manufacturing processes in real-time
- Supporting method development and data generation
- Implementing corrective actions to maintain quality standards
Quality Control
Designation: Officer / Technician
Qualification: B.Sc, B.Pharm, Diploma, ITI, HSC
Experience: 2–7 years
Responsibilities:
- Handling QC instruments: HPLC, Dissolution, GC, IR, UV
- Reviewing analytical documents and QC-QMS records
- Conducting material testing and maintaining logbooks
Packing Department
Designation: Supervisor / Technician
Qualification: B.Sc, Diploma, MHSC, SSC
Experience: 2–7 years
Responsibilities:
- Operating and supervising CAM/IMA/Blister/CVC/Cartonator machines
- Managing documentation related to packaging operations
- Ensuring compliance with QMS and GMP
Production Department
Designation: Officer / Sr. Officer / Executive
Qualification: M.Pharm, M.Sc, B.Sc
Experience: 4–10 years
Responsibilities:
- Working on Compression, Granulation & Coating equipment (Fette, Glatt, Ganson)
- Conducting in-process checks as per SOP and BPR/EME guidelines
- Performing calibration of IPQC instruments
- Line clearance activities in manufacturing
Pithampur – SEZ (Formulation)
Quality Assurance (IPQA)
Designation: Officer / Sr. Officer / Executive
Qualification: M.Pharm, M.Sc
Experience: 4–11 years
Responsibilities:
- Performing analytical method validation and verification
- Supporting method transfer activities
- Preparing protocols, reports, and worksheets
Quality Control
Designation: Officer
Qualification: B.Sc, M.Sc
Experience: 1–6 years
Responsibilities:
- Conducting analytical testing and documentation
- Supporting QC-QMS activities
Wardha (API)
Production
Designation: Officer / Sr. Officer
Qualification: Diploma (Chemical), B.Sc Chemistry, M.Sc Chemistry
Experience: 2–7 years
Responsibilities:
- Operating reactors and API manufacturing equipment
- Maintaining BMR/HPR documentation
- Managing intermediates and process operations
Trainees (Freshers)
Qualification: Diploma, B.Sc Chemistry, M.Sc Chemistry
Responsibilities:
- Supporting API manufacturing workflows
- Learning plant operations and documentation practices
Quality Control
Designation: Chemist / Technician
Qualification: M.Sc, B.Sc Chemistry
Experience: 2–7 years
Responsibilities:
- Handling instruments like HPLC, GC, IR, UV
- Reviewing QC documentation and QMS tasks
Engineering – Instrumentation & Utility
Designation: Supervisor / Technician
Qualification: Diploma, ITI
Experience: 2–7 years
Responsibilities:
- Operating and maintaining instrumentation systems
- Supporting utility operations in the API plant
Eligibility / Qualifications
- Qualifications required: ITI, Diploma, HSC, B.Sc, B.Pharm, M.Pharm, M.Sc
- Experience range: Freshers to 10 years (depending on role)
- Candidates with regulated plant experience (USFDA, MHRA) preferred
- Candidates who attended Ipca interviews in the last 6 months should not apply
- Must be open to shift duties
Relevant Courses: B.Sc Chemistry, B.Sc Microbiology, B.Sc Biotechnology, B.Pharm, M.Pharm, M.Sc Analytical Chemistry, M.Sc Organic Chemistry, Diploma Chemical Engineering, ITI, SSC/HSC.
Location & Salary
Walk-in Venue: Lemon Tree Hotel, Opp. Parsi Agyari, Sarod, Sayajiganj, Vadodara, Gujarat 390007
Date: 21 December 2025 (Sunday)
Time: 09:00 AM to 04:00 PM
Salary is not disclosed. Compensation will depend on qualification, experience, and interview performance.
Application Process
This is a direct walk-in recruitment drive. Candidates must carry the following:
- Updated Resume
- Passport-size photograph
- 10th / 12th / ITI / Diploma / Graduation / Post-graduation certificates
- All experience and joining letters from previous companies
- Current company CTC details, appointment letter, and pay slips
Important Note: Ipca does not charge any application fee.
FAQs
1. Can freshers apply?
Yes, freshers can apply for trainee roles at the Wardha API unit.
2. Are shift duties required?
Yes, candidates must be open to rotational shifts.
3. Is experience in regulated plants mandatory?
Not mandatory, but highly preferred.
4. What certifications must I bring?
All academic and experience certificates are mandatory.
5. Is this interview online?
No. This is strictly a walk-in interview.
Summary Table
| Company | Ipca Laboratories Ltd. |
|---|---|
| Vacancies | Multiple across QA, QC, Production, Packing, Engineering |
| Required Education | ITI, Diploma, HSC, B.Sc, B.Pharm, M.Pharm, M.Sc |
| Experience | Freshers to 10 years |