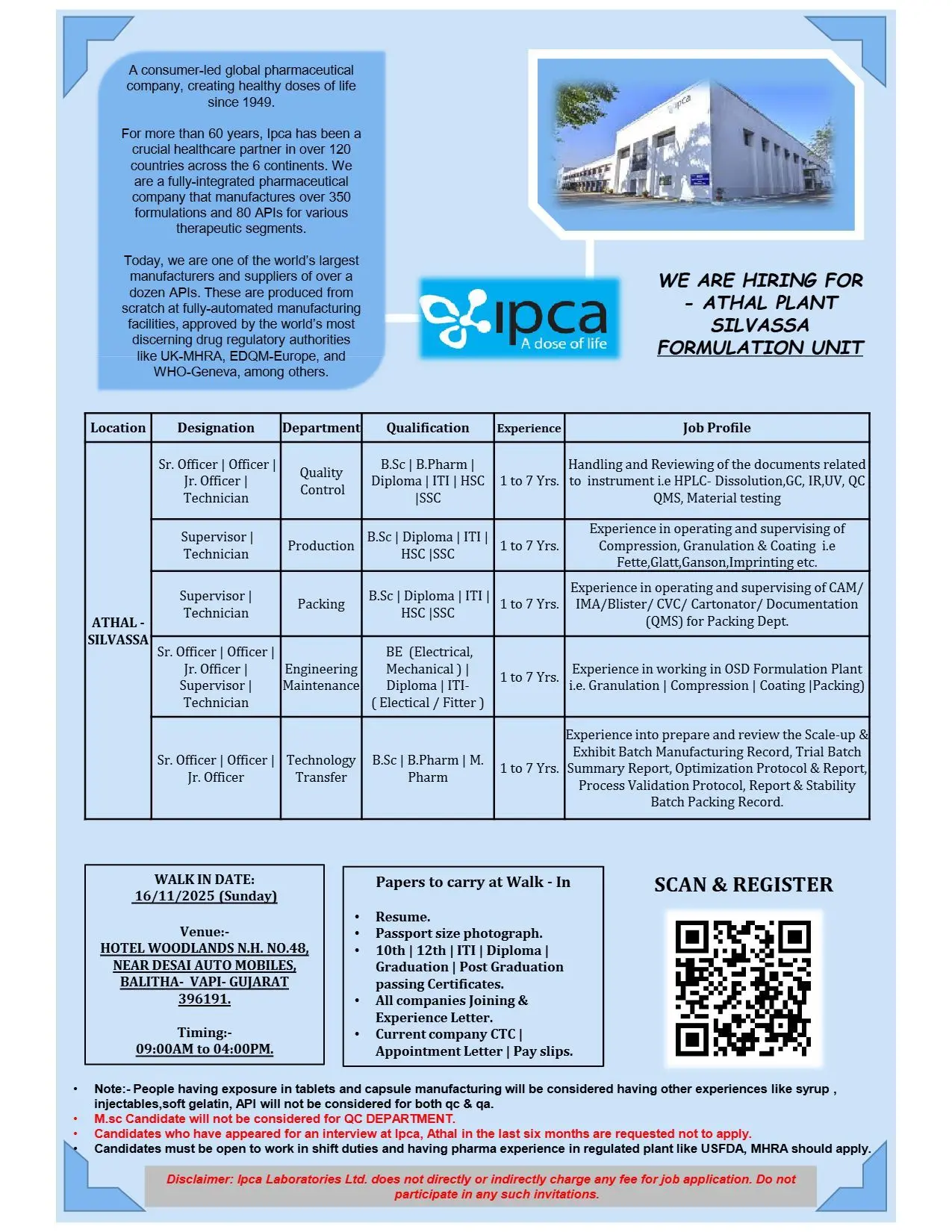
Ipca Laboratories walk-in QC, Production & Packing

- Company Overview
- Job Role & Responsibilities
- Department: Quality Control (QC)
- Department: Production (OSD)
- Department: Packing
- Department: Engineering & Maintenance
- Department: Technology Transfer (TT)
- Eligibility / Qualifications
- Location & Salary
- Application Process
- Key Highlights (Why Join Ipca Laboratories)
- FAQs
- EEAT & Trustworthiness Compliance
Ipca Laboratories QC, Production & Packing Openings – Vapi (16 Nov 2025)
B.Pharm/B.Sc/Diploma/ITI vacancies in QC, Production, Packing & Engineering at Ipca Athal Plant – Walk-in 16 Nov 2025, Vapi, Gujarat.
Ipca Laboratories Ltd., a global pharmaceutical leader known for its high-quality formulations and APIs, is conducting a walk-in drive for multiple departments at its Athal Plant, Silvassa on 16th November 2025 (Sunday). The company is seeking qualified professionals with 1–7 years of experience in OSD (Oral Solid Dosage) formulations for roles across Quality Control, Production, Packing, Engineering, and Technology Transfer departments.
Company Overview
Founded in 1949, Ipca Laboratories Ltd. is a globally integrated pharmaceutical company present in over 120 countries across six continents. Ipca is recognized as one of the world’s largest manufacturers of APIs and formulations, producing over 350 dosage forms and 50 APIs across various therapeutic segments.
Ipca’s world-class manufacturing facilities are approved by international regulatory bodies such as UK-MHRA, EDQM (Europe), and WHO-Geneva. The company’s focus on innovation, compliance, and sustainable healthcare solutions has made it a trusted partner in global healthcare.
Joining Ipca offers professionals an opportunity to work in a fully automated, regulatory-approved facility that values precision, integrity, and growth.
Job Role & Responsibilities
Department: Quality Control (QC)
Positions: Sr. Officer, Officer, Jr. Officer, Technician
Qualification: B.Sc, B.Pharm, Diploma, ITI, HSC/SSC
Experience: 1–7 years
Key Responsibilities:
- Handle and review analytical documents related to QC instruments (HPLC, Dissolution, GC, IR, UV).
- Conduct material testing and manage QC QMS documentation.
- Ensure compliance with cGMP, data integrity, and audit readiness.
- Perform calibration, maintenance, and validation of analytical equipment.
Department: Production (OSD)
Positions: Supervisor, Technician
Qualification: B.Sc, Diploma, ITI, HSC/SSC
Experience: 1–7 years
Key Responsibilities:
- Operate and supervise machines for Compression, Granulation, and Coating (Fette, Glatt, Ganson, Imprinting).
- Monitor production parameters, maintain BMR documentation, and ensure batch quality.
- Follow GMP and safety standards during production activities.
Department: Packing
Positions: Sr. Officer, Officer, Supervisor, Technician
Qualification: HSC, SSC, B.Sc, Diploma, ITI
Experience: 1–7 years
Key Responsibilities:
- Operate and monitor CAM, IMA, Blister, CVC, Cartonator packing lines.
- Ensure packaging material traceability and documentation (QMS).
- Perform online checks and maintain equipment hygiene and compliance.
Department: Engineering & Maintenance
Positions: Jr. Officer, Supervisor, Technician
Qualification: BE (Electrical/Mechanical), Diploma, ITI (Electrical/Fitter)
Experience: 1–7 years
Key Responsibilities:
- Maintain and troubleshoot utilities and process equipment across OSD units.
- Conduct preventive and breakdown maintenance for Granulation, Compression, Coating, and Packing areas.
- Maintain records as per GMP and safety guidelines.
Department: Technology Transfer (TT)
Positions: Sr. Officer, Officer, Jr. Officer
Qualification: B.Sc, B.Pharm, M.Pharm
Experience: 1–7 years
Key Responsibilities:
- Prepare and review Scale-up and Exhibit Batch Manufacturing Records.
- Handle Trial Batch Summary Reports, Optimization Protocols, and Process Validation documentation.
- Coordinate with R&D, QA, and Production during new product transfers.
- Support stability batch packing and regulatory documentation.
Eligibility / Qualifications
Required Education: B.Pharm, M.Pharm, B.Sc, Diploma, ITI, HSC/SSC, BE (Electrical/Mechanical)
Relevant Courses (comma-separated):
B.Pharm, M.Pharm, B.Sc Chemistry, B.Sc Industrial Chemistry, Diploma Mechanical, Diploma Electrical, ITI Fitter, ITI Electrician, BE Mechanical, BE Electrical, Diploma in Pharmaceutical Technology, PG Diploma in Industrial Quality Control
Experience: 1–7 years (depending on department and designation).
Preference: Candidates with OSD formulation experience only will be considered. Candidates with experience in Syrup, Injectables, Soft Gelatin, or API manufacturing are not eligible.
Location & Salary
Location: Ipca Laboratories Ltd., Athal Plant, Silvassa
Walk-in Venue: Hotel Woodlands, NH-48, Near Desai Automobiles, Ralitha, Vapi, Gujarat – 396191
Walk-in Date: 16th November 2025 (Sunday)
Time: 09:00 AM to 04:00 PM
Salary: As per industry standards; commensurate with experience and current CTC.
Shifts: Candidates must be open to working in rotational shifts.
Application Process
Documents to Carry:
- Updated Resume
- Passport-size Photograph
- Educational Certificates (10th, 12th, ITI, Diploma, Graduation, Post-Graduation)
- Joining & Experience Letters from previous employers
- Current Company CTC structure, Appointment Letter, and last three months’ Pay Slips
Registration: Candidates can scan the QR code (shared by HR) for pre-registration before attending the walk-in.
Note: Candidates interviewed at Ipca Athal within the last six months are not eligible to reapply.
Contact: Visit the official Ipca website for details or email HR (if applicable).
Key Highlights (Why Join Ipca Laboratories)
- Opportunity to work with a globally recognized, MHRA-approved pharmaceutical manufacturer.
- Exposure to large-scale OSD manufacturing and automated technology.
- Stable, long-term career growth in production, QC, and technical operations.
- Transparent, ethical recruitment process (no fees or intermediaries).
FAQs
Q: Who can attend this walk-in drive?
A: Candidates with relevant qualifications and 1–7 years of experience in OSD formulation plants.
Q: Can candidates from API or injectable backgrounds apply?
A: No, only OSD formulation experience is accepted.
Q: What documents should I bring?
A: Resume, educational certificates, experience letters, CTC documents, and ID proofs.
Q: Are freshers eligible?
A: No, this drive is for experienced professionals only.
Q: Is there a registration process?
A: Yes, scan the QR code provided in the HR notice before attending.
Q: Are there any recruitment charges?
A: No, Ipca Laboratories never charges fees for job applications.
EEAT & Trustworthiness Compliance
- Expertise: Detailed role-based descriptions ensure accuracy and domain relevance.
- Authoritativeness: Highlights Ipca’s 75-year legacy and global regulatory approvals.
- Trustworthiness: Clear disclaimers and transparent recruitment guidelines build candidate confidence.
Vertical Summary Table
+------------------------------+----------------------------------------------------------------+
| Company | Ipca Laboratories Ltd. |
+------------------------------+----------------------------------------------------------------+
| Vacancies | QC, Production, Packing, Engineering, Technology Transfer |
+------------------------------+----------------------------------------------------------------+
| Required Education | B.Pharm, M.Pharm, B.Sc, Diploma, ITI, BE (Mech/Electrical) |
+------------------------------+----------------------------------------------------------------+
| Experience | 1–7 years (OSD Formulation Only) |
+------------------------------+----------------------------------------------------------------+
| Walk-in Date & Venue | 16-Nov-2025 | Hotel Woodlands, NH-48, Vapi, Gujarat |
+------------------------------+----------------------------------------------------------------+