Lupin Limited Walk-in Production, QA, and QC

- B.Pharm, M.Pharm Openings – Lupin Limited Walk-in Drive, Pithampur
- Company Overview
- Walk-in Interview Details
- Job Role & Responsibilities
- Production Department (Ophthalmic, Hormonal, Nasal, Derma)
- Quality Control (QC)
- Quality Assurance (QA)
- Eligibility / Qualifications
- Location & Salary
- Application Process
- Why Join Lupin Limited
- FAQs
B.Pharm, M.Pharm Openings – Lupin Limited Walk-in Drive, Pithampur
Attend Lupin Limited’s Walk-in Interview in Pithampur for Production, QA, and QC roles. Apply with 2–10 years of experience in Pharma Manufacturing.
Lupin Limited, one of India’s top pharmaceutical companies, is conducting a Walk-in Interview for various departments including Production, Quality Assurance (QA), and Quality Control (QC) at its Pithampur facility, Madhya Pradesh. This recruitment drive is open for professionals experienced in Sterile, Hormonal, Nasal, Derma, and Ophthalmic formulations. Candidates with exposure to USFDA and MHRA regulatory environments are highly encouraged to apply.
Company Overview
Lupin Limited is a global pharmaceutical leader known for its innovation in generics, biosimilars, and specialty drugs. With a strong commitment to quality, Lupin’s manufacturing facilities are approved by leading regulatory authorities like USFDA, MHRA, TGA, and WHO. The company’s presence spans over 100 countries, providing affordable and high-quality medicines across therapeutic areas such as Cardiology, Diabetology, Respiratory, and Dermatology.
Lupin’s Pithampur site is a benchmark for excellence in manufacturing formulations, ensuring compliance with international quality standards. Joining Lupin means being part of a science-driven organization that fosters professional growth and rewards performance.
Walk-in Interview Details
Date: 8th November 2025
Venue: Lupin Limited, Pithampur, Madhya Pradesh
Time: From 9:30 AM onwards
Email for Queries: porichayindore@lupin.com
Prerequisites:
- Relieving letters from all previous organizations.
- Candidates must not have attended a Lupin interview in the past six months.
- Minimum 60% marks mandatory for candidates with less than 5 years of experience.
Job Role & Responsibilities
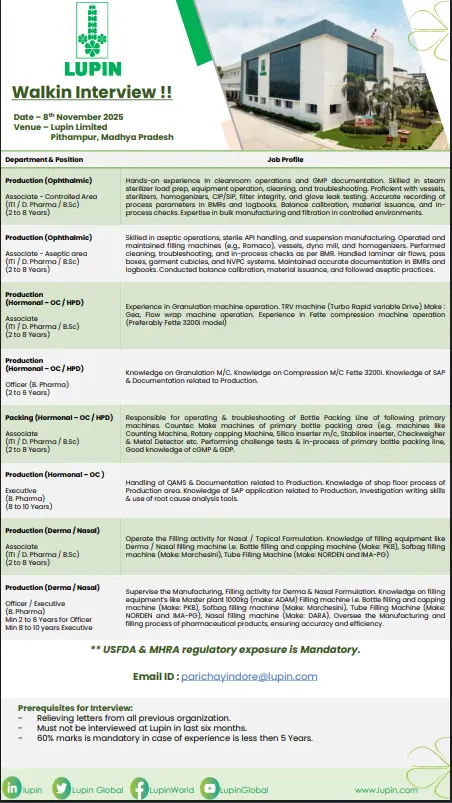
Production Department (Ophthalmic, Hormonal, Nasal, Derma)
Positions Available:
- Associate (ITI / D.Pharm / B.Sc) – 2 to 8 years of experience
- Officer / Executive (B.Pharm) – 2 to 10 years of experience
Key Responsibilities:
- Operate, monitor, and troubleshoot filling and manufacturing machines for Ophthalmic, Nasal, and Topical formulations.
- Manage bulk manufacturing, filtration, and sterilization activities in compliance with cGMP.
- Perform aseptic operations and monitor cleanroom environments.
- Operate bottle filling machines (PKB, Marchesini, NORDEN, DARA) and maintain equipment logs.
- Supervise production processes, ensuring regulatory compliance (USFDA, MHRA).
- Conduct in-process checks and maintain batch manufacturing records.
Preferred Skills:
- Strong understanding of GMP, GDP, and data integrity requirements.
- Prior experience in sterile formulation environments.
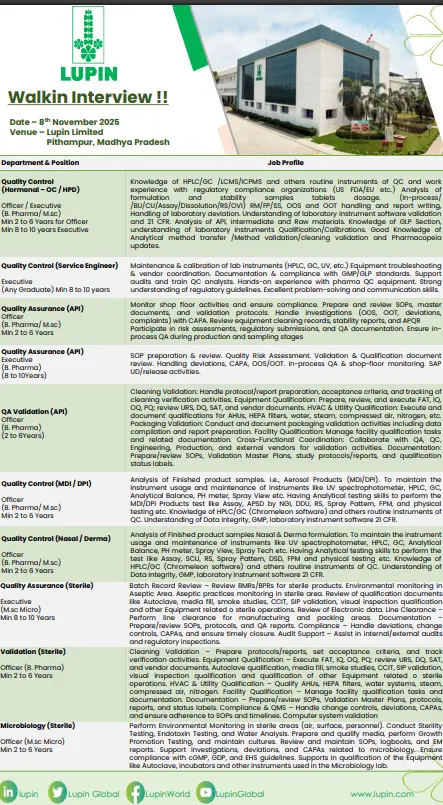
Quality Control (QC)
Positions: Officers / Executives (B.Pharm / M.Sc)
Experience: 2–10 Years
Departments: Hormonal, Nasal, Derma, MDI/DPI, Microbiology, Validation, Service Engineer
Key Responsibilities:
- Conduct analytical testing using HPLC, GC, LCMS, ICPMS, UV, and IR instruments.
- Perform finished product, raw material, and stability studies in compliance with GLP and GMP.
- Manage OOS/OOT investigations, data integrity reviews, and documentation control.
- Support method validation, cleaning validation, and analytical method transfer.
- Maintain laboratory instruments, perform calibrations, and support regulatory audits.
- Perform microbiological tests including sterility, endotoxin, and environmental monitoring for sterile areas.
Preferred Skills:
- Expertise in analytical method validation and laboratory compliance.
- Understanding of 21 CFR Part 11 and computerized system validation.
Quality Assurance (QA)
Positions: Officer / Executive (B.Pharm / M.Pharm)
Experience: 2–10 Years
Departments: APM, API, Sterile QA, Validation QA
Key Responsibilities:
- Review batch manufacturing records (BMR/BPR) and ensure documentation accuracy.
- Monitor shop floor compliance, line clearance, and aseptic practices.
- Prepare and review SOPs, validation protocols, CAPA, and deviation reports.
- Handle change controls, risk assessments, and equipment qualifications.
- Conduct cleaning validation, packaging validation, and facility qualification.
- Participate in internal and external audits, ensuring compliance with QMS and regulatory standards.
Preferred Skills:
- Exposure to USFDA/MHRA audit environments.
- Knowledge of HVAC, utility qualifications, and sterile area validations.
Eligibility / Qualifications
Educational Qualifications:
- ITI / D.Pharm / B.Sc / M.Sc / B.Pharm / M.Pharm
Experience Required:
- 2 to 10 years in Production, QA, or QC in a regulated pharmaceutical environment.
Relevant Courses (comma-separated):
B.Pharm, M.Pharm (Pharmaceutics), M.Sc (Microbiology, Analytical Chemistry), D.Pharm, ITI (Fitter/Electrical), Diploma in Mechanical Engineering, Certificate in GMP Compliance, Certificate in Analytical Method Validation, PG Diploma in Quality Assurance.
Preferred Industry:
- Exposure to sterile, hormonal, ophthalmic, or nasal formulation manufacturing.
Location & Salary
Location: Lupin Limited, Pithampur, Madhya Pradesh
Employment Type: Full-time
Compensation: Industry-standard salary with performance-based incentives, health benefits, and training opportunities.
Work Environment: USFDA & MHRA approved sterile and non-sterile formulation units.
Application Process
Walk-in Interview Date: 8th November 2025
Venue: Lupin Limited, Pithampur, Madhya Pradesh
Contact Email: porichayindore@lupin.com
Documents to Carry:
- Updated resume
- Recent photograph
- Educational and experience certificates
- Relieving letters from previous employers
Note: Candidates unable to attend may share their profiles via email.
Why Join Lupin Limited
- Opportunity to work with a globally renowned, research-driven pharmaceutical company.
- Exposure to advanced sterile manufacturing and regulatory audits (USFDA, MHRA).
- Collaborative work culture with a focus on innovation and quality.
- Competitive compensation, training programs, and career growth opportunities.
FAQs
Q1. What departments are open for recruitment at Lupin Pithampur?
A1. Openings are available in Production, Quality Assurance, and Quality Control across multiple formulation types.
Q2. What qualifications are required?
A2. ITI, D.Pharm, B.Pharm, M.Pharm, or M.Sc with relevant industry experience.
Q3. Is regulatory experience mandatory?
A3. Candidates with USFDA/MHRA audit exposure are preferred.
Q4. Can freshers apply?
A4. No, only candidates with 2–10 years of experience are eligible.
Q5. What is the interview location and date?
A5. Lupin Limited, Pithampur, Madhya Pradesh, on 8th November 2025.
Q6. What should candidates bring to the interview?
A6. Updated resume, certificates, photographs, and relieving letters.
| Company | Lupin Limited |
|---|---|
| Vacancies | Production, QA, QC, Microbiology, Validation |
| Required Education | ITI, D.Pharm, B.Pharm, M.Pharm, M.Sc |
| Experience | 2–10 Years |
| Location | Pithampur, Madhya Pradesh |