Macleods Walk-inQA, QC, Production,Engineering

- Company Overview
- Job Role & Responsibilities
- Eligibility / Qualifications
- Relevant Courses
- Location & Salary
- Application Process
- FAQs
- Summary Table
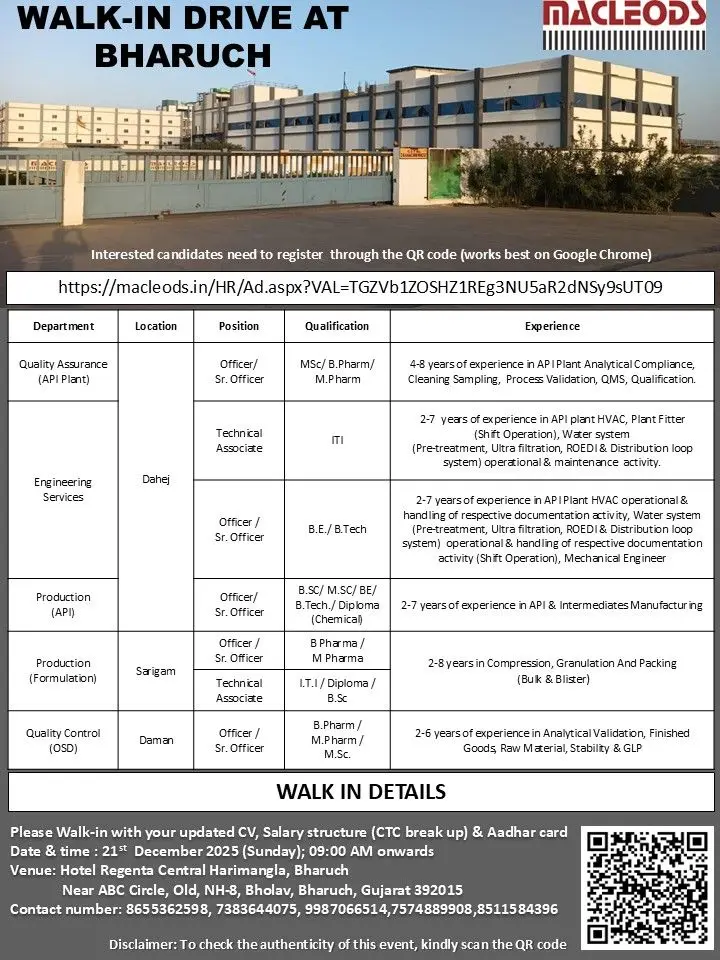
Macleods API & Formulation Walk-in for QA QC Production | Bharuch
Macleods hiring for QA, QC, Production, and Engineering at Dahej, Sarigam, Daman. Walk-in Bharuch on 21 Dec 2025. 2–8 years experience.
Macleods is conducting a large-scale walk-in drive in Bharuch for multiple openings across API and formulation units. These roles suit candidates with experience in quality assurance, quality control, production, and engineering services in regulated pharmaceutical manufacturing. The hiring covers vacancies at Dahej, Sarigam, and Daman locations.
Company Overview
Macleods Pharmaceuticals is one of India’s leading pharmaceutical organizations with a strong footprint in API and formulation manufacturing. The company operates globally compliant sites and offers structured training, advanced technology platforms, and exposure to regulatory inspections. Its plants are recognized for robust QMS systems, validated manufacturing processes, and strong documentation culture.
Job Role & Responsibilities
Macleods is hiring for multiple departments, each requiring domain-specific expertise.
1. Quality Assurance (API Plant) – Dahej
Position: Officer / Sr. Officer
Qualification: M.Sc / B.Pharm / M.Pharm
Experience: 4–8 years
Responsibilities:
- API plant analytical compliance activities
- Cleaning validation & sampling
- Process validation and qualification support
- QMS documentation, deviation handling, CAPA
2. Engineering Services (API Plant) – Dahej
Position: Technical Associate / Officer / Sr. Officer
Qualification: B.E / B.Tech
Experience: 2–7 years
Responsibilities:
- Handling HVAC operations and documentation
- Water system operations (RO, ROEDI, UF, pre-treatment, distribution loop)
- Shift operation support, equipment maintenance, troubleshooting
3. Production (API) – Dahej
Position: Officer / Sr. Officer
Qualification: B.Sc / M.Sc / B.E / B.Tech / Diploma (Chemical)
Experience: 2–7 years
Responsibilities:
- API and intermediates manufacturing activities
- Batch monitoring, equipment operation, and in-process checks
- Adherence to GMP and safety protocols
4. Production (Formulation) – Sarigam
Position: Officer / Sr. Officer
Qualification: B.Pharm / M.Pharm
Experience: 2–8 years
Responsibilities:
- Compression, granulation, packing (bulk & blister)
- Line clearance, BMR handling, and documentation
- Ensuring GMP compliance during formulation manufacturing
5. Quality Control (OSD) – Daman
Position: Officer / Sr. Officer / Technical Associate
Qualification: I.T.I / Diploma / B.Sc / B.Pharm / M.Pharm / M.Sc
Experience: 2–6 years
Responsibilities:
- Analytical method validation (AMV)
- Finished goods, raw materials, stability testing
- GLP compliance and documentation accuracy
Eligibility / Qualifications
- Mandatory pharmaceutical industry experience (API or formulation as applicable)
- Relevant degrees for each department as stated above
- Experience range: 2–8 years depending on role
Relevant Courses
B.Sc Chemistry, M.Sc Analytical Chemistry, M.Sc Organic Chemistry, B.Pharm, M.Pharm, B.E Chemical, B.Tech Mechanical, Diploma Chemical, ITI Maintenance.
Location & Salary
Walk-in Venue: Hotel Regenta Central Harimangla, Bharuch Circle, Old NH-8, Bholav, Bharuch, Gujarat – 392015
Date: 21 December 2025 (Sunday)
Time: 09:00 AM onwards
Vacancies are for Dahej, Sarigam, and Daman manufacturing sites. Salary will vary according to experience, skill level, and interview performance.
Application Process
Candidates must walk in with:
- Updated CV
- Salary structure / CTC breakup
- Aadhar card
- Supporting employment documents
Registration Link: https://macleods.in/HR/Ad.aspx?VAL=TGZVb1ZOSHZ1REg3NU5aR2dNSy9sUT09
Contact Numbers: 8655362598, 7383644075, 9987066514, 7574889908, 8511584396
FAQs
1. Is API experience mandatory for API roles?
Yes, relevant API plant experience is required.
2. Can candidates apply without registration?
Registration via QR code/link is recommended before attending.
3. What documents are essential?
Resume, CTC breakup, Aadhaar, and employment documents.
4. Are multiple plant locations hiring?
Yes—Dahej, Sarigam, and Daman.
5. Is GMP experience required?
Yes, roles require strong understanding of GMP and documentation.
Summary Table
| Company | Macleods Pharmaceuticals |
|---|---|
| Vacancies | QA, QC, Production, Engineering |
| Required Education | B.Sc, M.Sc, B.Pharm, M.Pharm, B.E, B.Tech, Diploma, ITI |
| Experience | 2–8 years depending on role |