Mepro Pharmaceuticals Hiring QC, RA, HR

- Company Overview
- Job Role & Responsibilities
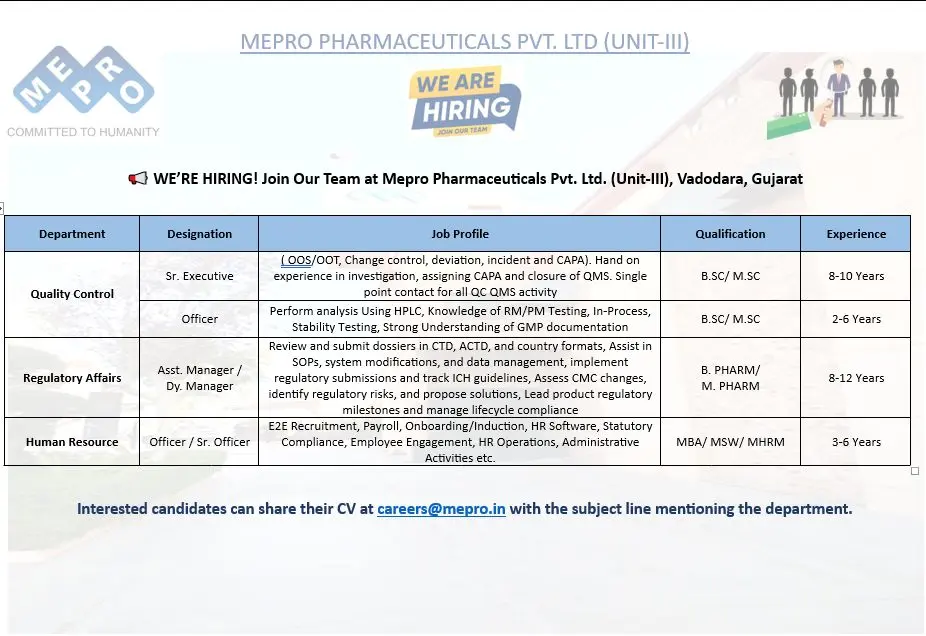
- Quality Control – Sr. Executive (QMS: OOS/OOT, Change Control, CAPA)
- Quality Control – Officer (HPLC/RM/PM/Stability)
- Regulatory Affairs – Asst. Manager / Dy. Manager
- Human Resource – Officer / Sr. Officer
- Eligibility / Qualifications
- Relevant Courses
- Location & Salary
- Application Process
- FAQs
- Are these roles for experienced candidates?
- Is QMS experience mandatory for QC Sr. Executive?
- Can freshers apply for any role?
- What dossier formats are required in RA?
- Are HR roles limited to manufacturing experience?
- Summary Table
B.Sc, B.Pharm QC & RA Openings | Mepro Pharmaceuticals Vadodara
Mepro Pharmaceuticals hiring for QC, RA, and HR roles in Vadodara. B.Sc, M.Sc, B.Pharm, M.Pharm, MBA candidates eligible.
Mepro Pharmaceuticals Pvt. Ltd. (Unit-III), Vadodara, is expanding its Quality Control, Regulatory Affairs, and Human Resources teams. These openings suit candidates with strong functional expertise and experience in pharma quality systems, regulatory submissions, laboratory operations, and HR processes.
Company Overview
Mepro Pharmaceuticals Pvt. Ltd. is a fast-growing manufacturer of formulations catering to domestic and international markets. The company operates with modern infrastructure, advanced analytical laboratories, and a strong quality management system. Its Vadodara Unit-III facility supports regulated-market manufacturing and offers career growth for professionals who value compliance, efficiency, and continuous improvement.
Job Role & Responsibilities
Quality Control – Sr. Executive (QMS: OOS/OOT, Change Control, CAPA)
- Lead investigations for OOS/OOT, deviations, incidents, and change controls
- Assign, track, and close CAPA in alignment with QMS requirements
- Act as the single point of contact for all QC-QMS activities
- Ensure timely documentation, compliance, and audit readiness
- Support continuous improvement initiatives within the QC department
Quality Control – Officer (HPLC/RM/PM/Stability)
- Perform analytical testing using HPLC and other QC instruments
- Conduct RM/PM testing, in-process checks, and stability analysis
- Ensure adherence to GMP documentation, data integrity, and SOPs
- Maintain analytical records, calibration logs, and sample management
Regulatory Affairs – Asst. Manager / Dy. Manager
- Review and submit dossiers in CTD, ACTD, and country-specific formats
- Assist in SOP development, system enhancements, and regulatory data management
- Implement regulatory submissions and track ICH guidelines
- Assess CMC changes, identify risks, and provide regulatory solutions
- Lead lifecycle management and achieve regulatory milestones for assigned products
Human Resource – Officer / Sr. Officer
- Manage end-to-end recruitment and onboarding processes
- Handle payroll, statutory compliance, and HR operations
- Coordinate employee engagement initiatives and administrative tasks
- Work with HRMS tools for data updates and process optimization
- Support induction programs and employee record management
Eligibility / Qualifications
- QC Sr. Executive: B.Sc / M.Sc, 8–10 years
- QC Officer: B.Sc / M.Sc, 2–6 years
- Regulatory Affairs Asst. Manager / Dy. Manager: B.Pharm / M.Pharm, 8–12 years
- HR Officer / Sr. Officer: MBA / MSW / MHRM, 3–6 years
Relevant Courses
B.Sc Chemistry, M.Sc Analytical Chemistry, B.Pharm, M.Pharm Regulatory Affairs, MBA HR, PG Diploma in QA/QC, Certification in QMS & CAPA.
Location & Salary
- Location: Mepro Pharmaceuticals Pvt. Ltd. (Unit-III), Vadodara, Gujarat
- Salary: Based on experience and internal grade structure
Application Process
Interested candidates may share their resume at:
- Email: careers@mepro.in
Mention your desired department in the subject line for faster processing.
FAQs
Are these roles for experienced candidates?
Yes. All positions require 2–12 years of relevant experience.
Is QMS experience mandatory for QC Sr. Executive?
Yes. Strong hands-on experience in OOS/OOT, CAPA, and investigations is essential.
Can freshers apply for any role?
No. These roles are for experienced professionals only.
What dossier formats are required in RA?
CTD, ACTD, and market-specific dossiers.
Are HR roles limited to manufacturing experience?
Pharma HR experience is preferred but not mandatory.
Summary Table
| Company | Mepro Pharmaceuticals Pvt. Ltd. (Unit-III) |
|---|---|
| Vacancies | QC Sr. Executive, QC Officer, RA Manager, HR Officer |
| Required Education | B.Sc, M.Sc, B.Pharm, M.Pharm, MBA |
| Experience | 2–12 years depending on role |
You must sign in to apply for this position.


